Durvalumab monotherapy appears safe and well-tolerated
Neoadjuvant and adjuvant monotherapy using the checkpoint inhibitor durvalumab is well-tolerated by patients with locally advanced renal cell carcinoma (RCC), but adding a second checkpoint inhibitor, tremelimumab, caused complications that forced some patients to discontinue the combination therapy.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Those are the findings of a phase Ib trial conducted by a team of Cleveland Clinic Cancer Center investigators and their collaborators and presented at the 2020 American Society of Clinical Oncology virtual annual meeting.
“In our trial, perioperative durvalumab seems safe in this patient population, but the addition of tremelimumab was associated with increased toxicity and rates of discontinuation,” says Cleveland Clinic Cancer Center medical oncologist Moshe Ornstein, MD, MA. Dr. Ornstein led the trial after former Cleveland Clinic Cancer Center oncologist Brian Rini, MD, joined Vanderbilt University Medical Center.
In a significant portion of patients who received durvalumab plus tremelimumab, high-dose steroids were needed to manage immune-related adverse events (AEs). The higher-than-expected therapy discontinuations due to AEs prompted suspension of the trial. The protocol-defined maximum tolerated doses of durvalumab and tremelimumab were not reached.
In explaining the rationale for this trial, Dr. Ornstein noted that there are currently no approved preoperative therapies for patients with locally advanced RCC prior to nephrectomy, and only one approved therapy, sunitinib, for patients with high-risk localized RCC after surgery. Sunitinib is a receptor protein-tyrosine kinase inhibitor that disrupts tumor angiogenesis by blocking the actions of vascular endothelial growth factor, as well as affecting tumor cell proliferation.
“From the metastatic RCC setting, we know that immune checkpoint inhibitors (ICIs) have excellent clinical activity in patients with metastatic kidney cancer, so we wanted to investigate the role of immunotherapy in patients with locally advanced kidney cancer and, specifically, not just giving it after surgery but also giving it before surgery,” he says.
Advertisement
The investigators hypothesized that delivering ICI therapy neoadjuvantly, while the tumor and neoantigens are still present, might increase the efficacy of these agents.
“The goal of this trial was to see whether this approach is safe, since we did not have safety data in the localized RCC setting for the combination of a PD-L1 inhibitor and a CTLA-4 inhibitor,” Dr. Ornstein adds.
Twenty-nine patients were enrolled in the trial and divided among three cohorts (1-3); cohort 2a was added after the rates of toxicity in Cohort 3 were found to be too high (Figure 1). Patients in each cohort received durvalumab or durvalumab plus tremelimumab before surgery and durvalumab monotherapy or durvalumab plus tremelimumab after surgery. The neoadjuvant dose was given a median of 7 days before surgery.
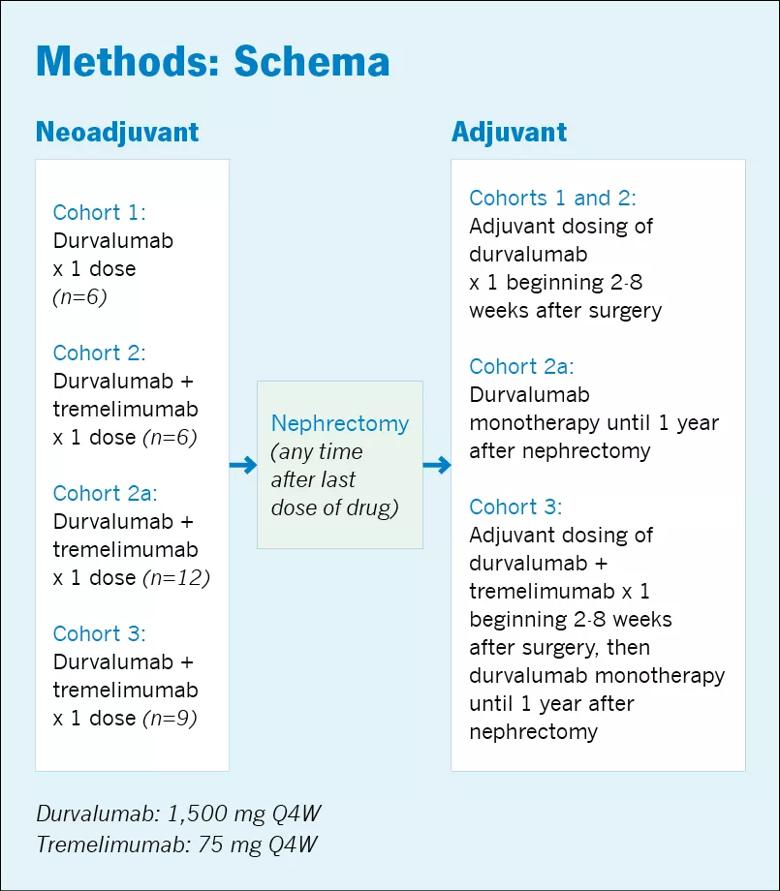
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6cf14dd2-5a2c-4222-a070-c709565c9e13/805x-Inset-ASCO-2020-Ornstein_jpg)
“These patients were very typical of what we would see in clinic and fairly representative of the patient population with locally advanced RCC,” says Dr. Ornstein, who adds that it is important to highlight the rates of immune-related AEs and rates of discontinuation secondary to AEs in this group of patients. “When you’re dealing with patients who have localized disease and are, in theory, curable just with surgery, even with a lower number of side effects, you are going to start to be concerned.”
In Cohorts 1 and 2, the treatment regimen was well-tolerated, with only 1 of 6 patients in each cohort experiencing a significant side effect. In Cohort 1, one patient experienced a Grade 4 AE (diabetic ketoacidosis) and in Cohort 2 one patient experienced a Grade 3 AE (thrombocytopenia). Of the 12 patients in both cohorts, one required high-dose steroid therapy and one had to discontinue therapy due to toxicity.
Advertisement
The rate of discontinuation was fairly high in Cohort 2a (38%). In Cohort 3, more than 50% of patients had to discontinue treatment due to AEs and 44% required high-dose steroids. The Grade 3 and 4 AEs in these cohorts included arthralgia/myalgia, paresthesia, elevated lipase/amylase, elevated alanine transaminase/aspartate transaminase and rashes.
“I think the take-home message here is that durvalumab by itself before and after surgery definitely appears safe,” says Dr. Ornstein, but that the addition of tremelimumab produced signs of toxicity. “Giving the combination treatment before and after nephrectomy looks worrisome, but the question is, is there still a role, like in Cohort 2, for giving the combination before the surgery, or adding one dose of the CTLA-4 antibody.”
Dr. Ornstein says there are larger ongoing trials, including RAMPART (Renal Adjuvant MultiPle Arm Randomised Trial) from the United Kingdom, that are further looking into the safety and efficacy of both agents in locally advanced RCC.
Dr. Ornstein’s team also will assess the long-term outcomes in patients treated with durvalumab and/or tremelimumab in the Cleveland Clinic trial.
“The clinical ongoing portion is looking at disease recurrence,” he says. Additionally, the trial will evaluate “the impact of these drugs on the tumor microenvironment and the correlatives between certain molecular and genomic findings and immune-related AEs.”
Advertisement
Advertisement

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors

Study shows significantly reduced risk of mortality and disease complications in patients receiving GLP-1 agonists

Structured interventions enhance sleep, safety and caregiver resiliency in high-acuity units