Phase 2 trials investigate sitagliptin and methimazole as adjuvant therapies
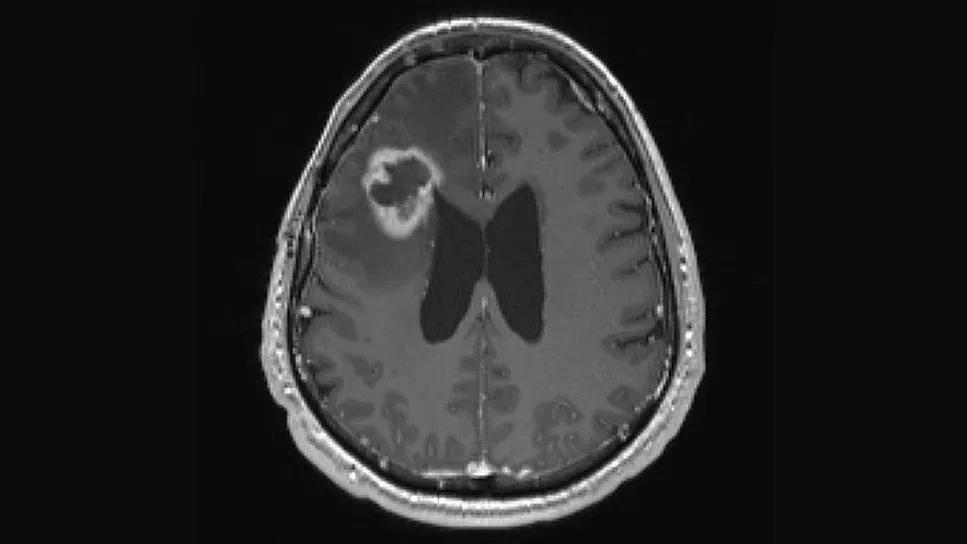
Two “window of opportunity” phase 2 clinical trials for patients with progressive glioblastoma are underway at Cleveland Clinic to assess safety, efficacy and tissue correlates with the use oftwo repurposed FDA-approved drugs in combination with chemotherapy.Both trials are designed for patients with progressive glioblastoma for whom a clinically indicated surgical resection is planned.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Abstracts describing the trials — one testing methimazole, used to treat hyperthyroidism, and the other evaluating sitagliptin, used to treat type 2 diabetes — were presented at the annual meeting of the Society for Neuro-Oncology (SNO), held in conjunction with the World Federation of Neuro-Oncology Societies in November 2025.
“Translational research performed at Cleveland Clinic has led us to recognize the potential for repurposing existing drugs to fight glioblastoma,“ says David Peereboom, MD, a staff medical oncologist with Cleveland Clinic’s Rose Ella Burkhardt Brain Tumor and Neuro-Oncology Center and principal investigator of both clinical trials. “These window-of-opportunity trials give us the unique opportunity to directly examine impacts of new therapies administered preoperatively, which allows us to measure drug concentrations in the brain tumor and the drug’s effect on the tumor microenvironment.”
Hypothyroidism increases the endogenous production of gaseous hydrogen sulfide (H2S), which Cleveland Clinic investigators have demonstrated inhibits growth of cultured glioblastoma cells and slows tumor progression in murine models. Additional evidence was found in human brain tissue, where H2S levels were found to be lower in glioblastoma than in normal brain.
“Our early research led us to hypothesize that boosting H2S levels could offer a novel therapeutic approach for glioblastoma,” Dr. Peereboom explains.
Because exogenous H2S cannot feasibly be given to people directly, the research team sought ways to promote endogenous H2S synthesis. The thyroid hormone inhibitor methimazole was found to safely and effectively increase production of H2S.
Advertisement
The single-arm Phase 2 and Pharmacodynamic Trial of Methimazole in Patients with Progressive Glioblastoma (NCT05607407) commenced in January 2023 and is estimated to complete accrual this year. Thus far, 15 patients have enrolled.
Oral methimazole is given for at least five days preoperatively and then restarted postoperatively with the addition of standard chemotherapy until disease progression.
Patients were originally started on methimazole 15 mg/day, with the most recent cohort taking 25 mg/day to enhance H2S production. Early results have found:
Progression-free survival at six months was 15%, better than expected from standard treatment, and overall survival at 12 months was 21%, about the same as expected with standard treatment.
“We are encouraged to see preliminary signs of efficacy associated with hypothyroidism without causing symptoms of hypothyroidism or worsened chemotherapy toxicities,” Dr. Peereboom concludes. “If our final results prove promising, we will embark on a larger trial.”
Extensive research at Cleveland Clinic has focused on understanding the role of myeloid-derived suppressor cells (MDSCs), immature cells that block cytotoxic T-cell immune responses, in glioblastoma. Their presence in glioblastoma represents a key mechanism of immunosuppression in the glioblastoma microenvironment, which in turn helps tumors evade the immune system and thereby resist cancer therapies. The investigators found that, in the setting of glioblastoma, MDSCs increase systemically and accumulate locally in the tumor, with higher levels being associated with a poorer prognosis.
Advertisement
Dr. Peereboom previously led research (JCI Insight.2019;4[22]:e130748) demonstrating that in patients with recurrent glioblastoma, presurgical reduction of circulating MDSCs with the chemotherapy drug capecitabine increases immune infiltration of tumor when subsequently treated with bevacizumab (an antiangiogenic medication used in recurrent glioblastoma). This finding suggested that efficacy of standard drug therapy might be improved by reduced levels of MDSCs.
Searching for less-toxic methods to suppress MDSCs, Cleveland Clinic investigators found that these cells require the enzyme dipeptidyl peptidase-4 (DPP-4) for overall function and brain entry. The antidiabetic drug sitagliptin, a DDP-4 inhibitor with low toxicity, showed good efficacy for reducing circulating MDSCs in preclinical models of glioblastoma.
This research laid the groundwork for a newly activated clinical trial described at the SNO annual meeting (NCT07003542). It will assess whether pre- and postoperative sitagliptin can reduce the number of MDSCs in the brain tumor and thereby reduce their immunosuppressive effects, enhancing the efficacy of standard chemotherapy.
The cohort will consist of 48 patients to be randomized to receive both pre- and postoperative treatment with sitagliptin (n = 36) or postoperative sitagliptin alone (n = 12). The latter patients will serve as a control group to assess baseline levels of CD8+ T cells in patients who have not received sitagliptin. Postoperatively, all patients will receive sitagliptin as well as standard chemotherapy until disease progression.
Advertisement
Endpoints include comparisons between the two groups in tumor CD8+ T-cell counts in blood and tumor tissue at the time of resection, rates of progression-free survival at six months and overall survival at 12 months, and safety. Systemic and tumor immunological signals will also be evaluated, as will radiomic features on MRI.
“In both trials, we hope that by giving the immune system a better chance to do its job, standard chemotherapy will be more effective and ultimately improve survival,” Dr. Peereboom says. “This research may one day lead to much-needed new adjuvant therapies for a very difficult-to-control cancer.”
Advertisement
Advertisement

Advances in genomics, spinal fluid analysis, wearable-based patient monitoring and more

Researchers use AI tools to compare clinical events with continuous patient monitoring

Combining dual inhibition with anti-PD1 therapy yielded >60% rate of complete tumor regression

Cleveland Clinic researchers pursue answers on basic science and clinical fronts
New research from Cleveland Clinic helps explain why these tumors are so refractory to treatment, and suggests new therapeutic avenues

Presurgical planning and careful consideration of pathology are key to achieving benefits
Study demonstrates its role in tumor lethality, raises prospect of therapeutic targets

Focused ultrasound is paired with ALA to utilize sonodynamic therapy to target cancer cells