Combining dual inhibition with anti-PD1 therapy yielded >60% rate of complete tumor regression
Cleveland Clinic researchers from the laboratory of Peiwen Chen, PhD, have developed a glioblastoma treatment strategy that shows a greater than 60% complete tumor regression rate in preclinical models. The strategy is a combination therapy consisting of anti-PD1 immunotherapy and two compounds that block macrophage and microglia infiltration.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The investigators’ results, published in the Journal of Clinical Investigation (2024;134[22]:e178628), demonstrated that the triple combination therapy provided long-lasting protection from future tumors, providing hope that new options can be developed for glioblastoma.
“We found that targeting both macrophages and microglia produces strong antitumor effects and results in complete tumor regression in over 60% of animals when combined with anti-PD1 therapy in PTEN-deficient mouse models of glioblastoma,” says Dr. Chen, associate staff in the Department of Cancer Biology in Cleveland Clinic Lerner Research Institute. “These findings suggest a translational triple therapeutic strategy for this deadly cancer.”
Tumor-associated macrophages and microglia (TAMs) play a crucial role in therapy resistance in glioblastoma, promoting tumor progression and suppressing tumor-fighting immune cells. Glioblastoma tumors are recognized as being highly infiltrated with immunosuppressive TAMs, which is believed to explain why they do not respond well to immunotherapies that use a patient’s own immune system.
Whereas many developers of therapeutics have tried — without success — to block macrophage and microglia function in glioblastoma, Dr. Chen notes that no one had previously tried to block both macrophages and microglia at the same time.
This research gap helped spurred the work reported in his lab’s new study publication. The work built on previous research by his team showing that glioblastoma tumors can have unique TAM signatures based on the specific genetic mutations driving the cancer. These context-dependent TAM profiles require context-dependent TAM therapy.
Advertisement
For example, the team had previously found that mutations in the tumor suppressor gene PTEN can activate a different gene, called LOX, that promotes macrophage infiltration into the tumor without affecting microglia. They had similarly found that a different driver of glioblastoma, the circadian regulator CLOCK, activates a gene called OLFML3, which in turn promotes immunosuppressive microglia but not macrophages.
“Simply blocking LOX in the context of PTEN-associated tumors or blocking OLFML3 in CLOCK-associated tumors is not enough to cure glioblastoma on its own,” Dr. Chen observes.
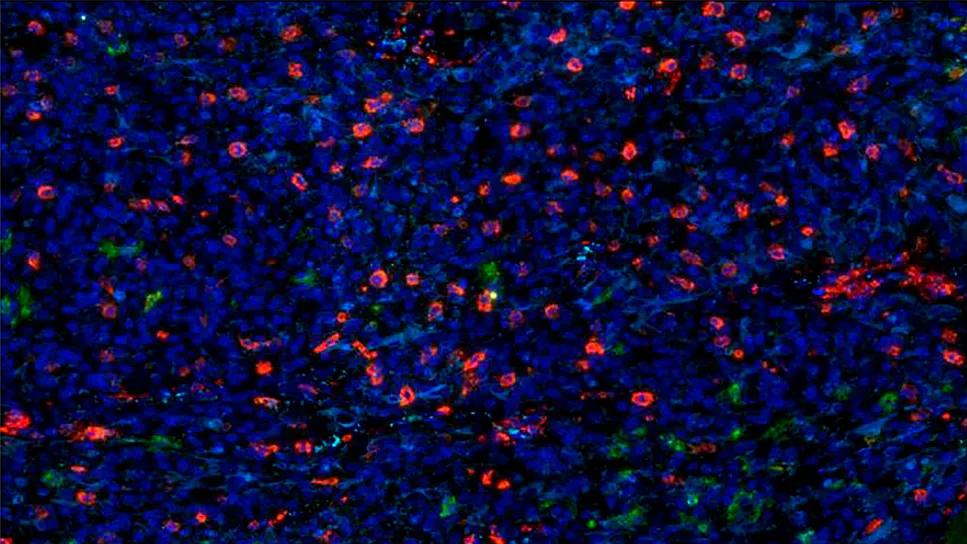
To better understand the relationship among immune cells in brain tumors, he and his team analyzed single-cell RNA sequencing data from the tumors of human glioblastoma patients. The researchers were surprised to see that macrophage-specific gene signatures (i.e., LOX) and microglia-specific gene signatures (i.e., CLOCK) were never activated at the same time. Image analyses also showed that macrophages and microglia did not overlap in a tumor.
“Because macrophages and microglia are both immune cells in the brain tumor microenvironment, and because they can both help tumor progression and induce immunosuppression, people think these two cell types get along like sisters,” Dr. Chen says. “However, what we found is that they actually compete like rivals. When you stop microglia from working in the tumor, tumor-associated macrophages explode in numbers. The opposite is true, too — blocking macrophages increases microglia density in the tumor. This creates a net-zero effect. Understanding this competitive relationship could be the key to developing better therapies by targeting both macrophages and microglia.”
Advertisement
To begin to explore that concept, his team tested the inhibition of genes tied to microglia (OLFML3) and macrophages (LOX) independently and in combination with anti-PD1 immunotherapy (which generally fails to combat glioblastoma on its own) in mouse models of glioblastoma. Blocking the genes individually led to a moderate lifespan increase. Notably, the triple therapy (LOX inhibition + CLOCK-OLFML3 inhibition + anti-PD1 therapy) was highly effective against PTEN-deficient glioblastoma, as tumors disappeared in over 60% of animals. The triple therapy even protected the cured animals when the researchers tried to reintroduce tumors.
“This research has revealed a mechanism underlying the inverse relationship between macrophages and microglia in the glioblastoma tumor microenvironment,” Dr. Chen concludes. “These findings can offer translational guidance for the design of a potentially effective treatment strategy involving dual targeting of macrophages and microglia in combination with anti-PD1 immunotherapy. We look forward to building on these insights.”
Image at top: Multiplex sequential immunofluorescence showing the distribution of macrophages and microglia in the tumor of a patient with glioblastoma. Reprinted from Journal of Clinical Investigation (2024;134[22]:e178628) under terms of the Creative Commons Attribution 4.0 International License.
Advertisement
Advertisement

Phase 2 trials investigate sitagliptin and methimazole as adjuvant therapies

Advances in genomics, spinal fluid analysis, wearable-based patient monitoring and more

Researchers use AI tools to compare clinical events with continuous patient monitoring

Cleveland Clinic researchers pursue answers on basic science and clinical fronts
New research from Cleveland Clinic helps explain why these tumors are so refractory to treatment, and suggests new therapeutic avenues

Presurgical planning and careful consideration of pathology are key to achieving benefits
Study demonstrates its role in tumor lethality, raises prospect of therapeutic targets

Focused ultrasound is paired with ALA to utilize sonodynamic therapy to target cancer cells