Study demonstrates its role in tumor lethality, raises prospect of therapeutic targets
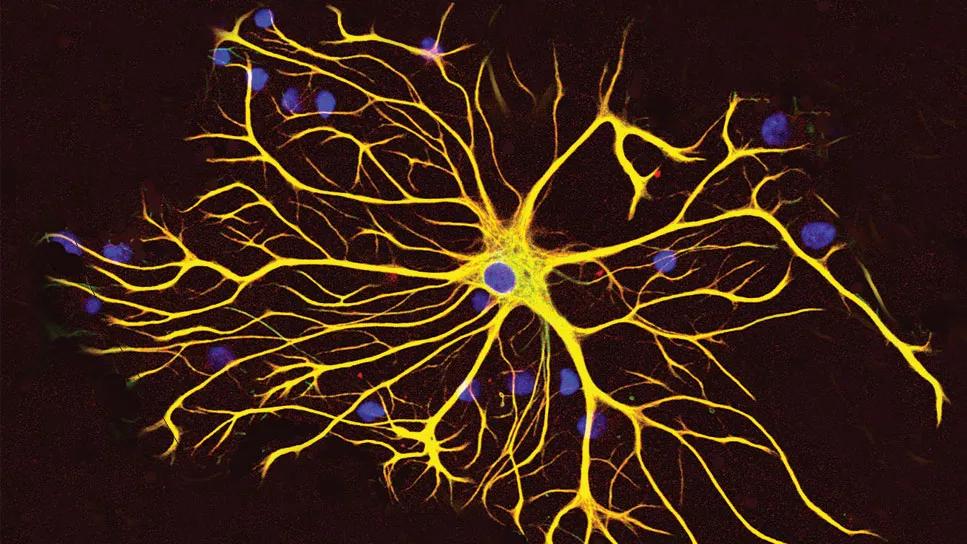
Glioblastoma cells use mitochondria from the central nervous system (CNS) to grow and form more aggressive tumors, according to a new Cleveland Clinic-led investigation published in Nature Cancer.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The research shows that it is common for healthy astrocytes ― a type of glial cell with important functions in the CNS ― to transfer their energy-producing organelles to glioblastoma cells. This process makes glioblastoma more lethal and the tumors more likely to grow. The researchers found that acquiring mitochondria boosted energy production and amplified cancer stem cells, making the latter even more difficult to treat.
“Defining the complex interactions glioblastoma cells have with the brain and nervous system is critical for developing new treatments,” says co-senior author Justin Lathia, PhD, the Melvin H. Burkhardt Endowed Chair for Neuro-Oncology Clinical Research and staff in the Department of Cardiovascular & Metabolic Sciences in Cleveland Clinic’s Lerner Research Institute. “We have shown that mitochondria transfer is a prevalent phenomenon in glioblastoma. We knew this type of transfer was theoretically possible, but we didn’t know how relevant and dangerous it was in brain tumors.”
Cancers, including glioblastoma, are resilient in part because of resources in the environment, capitalizing on the body’s natural defenses to protect cancer cells. Determining how cancer cells interact with healthy cells to survive may allow researchers to design new treatments to block cancer from growing or resisting treatment.
The current study investigated mitochondria transfer in glioblastoma, the most common and deadly type of primary brain tumor. Mitochondria transfer between cells is part of an emerging type of cell-to-cell interaction that is still being characterized. Its functional implications on tumor biology have been poorly understood.
Advertisement
Using human cell culture and mouse tumor cells, Dr. Lathia and colleagues from Cleveland Clinic and several other institutions in the U.S. and Europe demonstrated that horizontal mitochondria transfer from astrocytes enhances tumorigenesis in glioblastoma.
They found that the transfer relies on networks of intercellular connections between glioblastoma cells and astrocytes facilitated by growth-associated protein 43 (GAP43), which plays a role in neuron axon regeneration and astrocyte reactivity.
The researchers showed that transfer of astrocyte mitochondria to glioblastoma cells promotes mitochondrial respiration and upregulation of metabolic pathways implicated in cell proliferation. This boosts cell cycle progression and supports cancer stem cell properties including self-renewal and tumorigenicity.
“Our findings reveal a host-tumor interaction that drives proliferation and self-renewal of cancer cells, providing opportunities for therapeutic development,” the researchers write in their study report. They note their findings ultimately might prove applicable to tumors outside the CNS.
“Cancer ― and cancer treatment ― does not exist in a vacuum,” Dr. Lathia observes. “We are not just treating and researching the tumors alone but are instead tapping into a diverse ecosystem. Further research into this pathway can identify new strategies for treating glioblastoma while also holding potential to improve understanding of other types of cancer.”
Advertisement
Advertisement

Phase 2 trials investigate sitagliptin and methimazole as adjuvant therapies

Advances in genomics, spinal fluid analysis, wearable-based patient monitoring and more

Researchers use AI tools to compare clinical events with continuous patient monitoring

Combining dual inhibition with anti-PD1 therapy yielded >60% rate of complete tumor regression

Cleveland Clinic researchers pursue answers on basic science and clinical fronts
New research from Cleveland Clinic helps explain why these tumors are so refractory to treatment, and suggests new therapeutic avenues

Presurgical planning and careful consideration of pathology are key to achieving benefits

Focused ultrasound is paired with ALA to utilize sonodynamic therapy to target cancer cells