Investigations on platelet/aneurysm interactions lead to $2.6 million NIH grant
As platelets are jostled by turbulent blood flow within an abdominal aortic aneurysm (AAA), they are activated to secrete factors that remodel and weaken the vessel wall. However, new research shows that blocking a newly identified olfactory membrane receptor reduces this harmful platelet reactivity. The finding raises the prospect of therapeutically targeting the olfactory receptor — perhaps with a molecule that is the active ingredient in spearmint extract — to stop AAA progression and prevent aortic rupture.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The newly characterized receptor and its contributions to AAA growth were described in a study published in the Journal of Clinical Investigation (2022 Mar 24;e152373) involving investigations on human tissue and mouse models of AAA, as well as an ex vivo system to replicate disturbed blood flow in aneurysmal arteries.
The work is the result of nearly six years of investigation into mechanisms of the interaction between platelets and vessel walls by a team led by Scott Cameron, MD, PhD, Section Head of Vascular Medicine at Cleveland Clinic. The findings led to a recent five-year, $2.6 million National Institutes of Health (NIH) grant that will allow Dr. Cameron’s group to extend their research in AAA physiology with the aim of developing novel methods to identify AAA risk and potential pharmacotherapies.
“Platelets have emerged as circulating biosensors, as they are associated with proteins that are useful for identifying aneurysms and determining their rate of progression,” Dr. Cameron explains. “In addition, the olfactory platelet receptor we discovered offers a possible target for developing the first medical therapy to treat aneurysms.”
Platelet aggregates and thrombi can be found in aneurysmal arterial segments, suggesting a possible role of platelets in AAA etiology and progression. More evidence of a platelet/aneurysm link came in a 2020 research letter published in the Journal of the American College of Cardiology by Dr. Cameron’s team in conjunction with Cleveland Clinic vascular surgeon Sean Lyden, MD, showing that long-term antiplatelet medication use confers protection from AAA development, dissection and rupture. The finding was based on interrogation of the U.S. National Inpatient Sample, which included 3.9 million patients with documented use of antiplatelet agents.
Advertisement
Yet antiplatelet therapy is only partially effective, Dr. Cameron notes, leading him to suspect that other mechanisms are at play in aneurysm growth and driving him to pursue further investigations in platelet physiology.
The research group observed that unlike platelets from healthy individuals, platelets in people with cardiovascular disease and relevant animal models are enriched in activated metalloproteinases (MMPs). Platelets from patients with AAAs are biomechanically activated in conditions of disrupted blood flow (i.e., within an aneurysm), causing them to secrete a specific MMP — namely, MMP9 (Figure). This research is the first to identify bidirectional interactions between circulating platelets and the aorta.
“Platelets actually communicate with the vessel wall via MMP9 and other proteins that mediate remodeling, ultimately determining AAA progression,” Dr. Cameron says. “Such proteins are potential candidates for biomarkers to diagnose and monitor AAAs.”
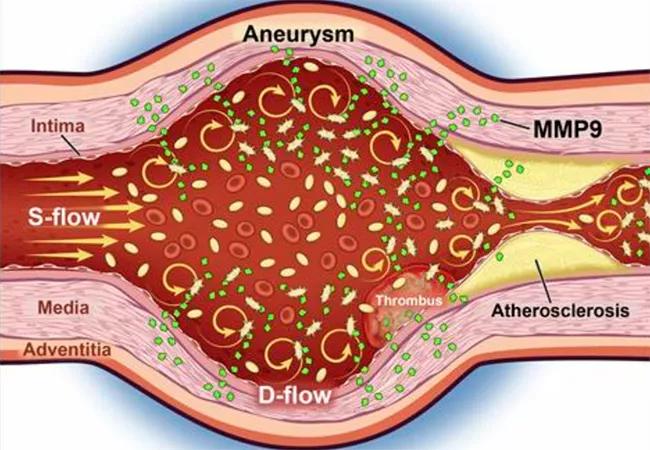
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7d91f588-61a9-44f4-b13c-73ef03a8b42f/21-HVI-2500366-CQD-650x450-1_jpg)
Figure. As blood enters an aneurysm, steady laminar flow (S-flow) becomes disturbed (D-flow), causing platelets to become biomechanically activated, releasing matrix metalloproteinase 9 (MMP). This results in further remodeling and progression of aneurysmal disease. Reprinted from Morrell et al., Journal of Clinical Investigation (2022 Mar 24;e152373).
The research team also discovered an olfactory receptor that binds odors on circulating platelets — OR2L13 — and which is more common on platelets in patients with AAAs. Dr. Cameron notes that olfactory receptors for cells other than those with a role in the sense of smell have been reported in only a few other papers and have been implicated in a variety of functions, from weight control to airway smooth muscle spasm, blood pressure regulation and atherosclerosis. Recently, there has been a surge of interest in olfactory receptors because they appear to be associated with blood clotting during COVID-19 infection.
Advertisement
Dr. Cameron’s research team screened for substances that bind to OR2L13 and found that carvone, the active ingredient in spearmint, and structurally related chemicals can be potent inhibitors of platelet activation. Specifically, they found that these chemicals reduce platelets’ release of MMP9, ultimately slowing AAA growth, lowering aortic rupture risk and preventing blood clotting. Other odorant chemicals were discovered that regulate the OR2L13 receptor, increasing thrombi formation.
The group’s NIH grant will enable them to further explore aneurysms in murine models. Mechanisms of AAA formation and progression are only beginning to be understood, and there are currently no pharmacotherapies available to limit AAA growth.
“Most AAAs remain asymptomatic until they rupture, which is associated with a 90% out-of-hospital mortality rate,” Dr. Cameron says. “Ultimately, we aim to develop novel diagnostic and pharmaceutical strategies so that aneurysmal disease can become a more manageable and less risky condition.”
He describes his team as an effective collaboration of highly specialized individuals from multiple institutions that includes experts in vascular medicine, cardiology, vascular surgery, pathology, biomedical genetics and medicinal chemistry, including Shaun Stauffer, PhD, Director of the Center for Therapeutics Discovery in Cleveland Clinic’s Lerner Research Institute.
Dr. Cameron says the broad objective of the team’s current research is twofold:
Advertisement
“This project offers the promise of the first medical therapy to treat AAAs,” Dr. Cameron notes. “We anticipate that our discoveries will also have broad clinical application to other conditions not directly studied in our investigations. These include peripheral and coronary artery disease as well as arterial aneurysmal disease in the cerebrovascular and peripheral circulation.”
“These findings from Dr. Cameron’s team are unique and exciting,” adds Dr. Lyden, Chair of Vascular Surgery at Cleveland Clinic. “With the largest volume of aortic cases in the United States, we hope we can someday apply these findings — and offer a new medical therapy — to future patients.”
Advertisement
Advertisement
First full characterization of kidney microbiome unlocks potential to prevent kidney stones
Researchers identify potential path to retaining chemo sensitivity
Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time
Investigators are developing a deep learning model to predict health outcomes in ICUs.
Preclinical work promises large-scale data with minimal bias to inform development of clinical tests
Cleveland Clinic researchers pursue answers on basic science and clinical fronts
Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches
Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology