Preclinical studies point to antitumor activity of the ERM inhibitor NSC668394
Treatment with NSC668394, a small molecule pharmacophore, reduced the viability and proliferation of rhabdomyosarcoma (RMS) cells and induced cell apoptosis, according to a preclinical study conducted by a group of Cleveland Clinic investigators. Study findings, published in Sarcoma, highlight a potential novel therapeutic strategy in RMS based on inhibition of ezrin-radixin-moesin (ERM) phosphorylation.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“This is the first study looking at targeting ezrin using a small molecule pharmacological approach in RMS, although the small molecule that we used — NSC668394— was identified and tested in osteosarcoma by another group,” says Cleveland Clinic investigator Neetu Gupta, PhD, director of the Center of Excellence in Lymphoid Malignancies Research. “Our findings showed that there is a beneficial effect of targeting ERM proteins with this agent in RMS.”
Dr. Gupta explains that, although a rare type of cancer, RMS is the most common pediatric soft tissue sarcoma. With approximately 350 new cases diagnosed per year, it accounts for 5-10% of all pediatric cancers.
“RMS is sort of an ‘underdog’ among pediatric cancers…there are not that many new therapies available,” she says. “The disease course really depends on the histological subtype and fusion between PAX3/7 and FOXO1 genes.” Dr. Gupta further explains that fusion-positive sarcomas have poor prognosis. In terms of histological subtypes, alveolar RMS (ARMS) is associated with worse prognosis compared to embryonal RMS (ERMS); however, fusion-positive status may change this. ERMS is approximately three times more common than ARMS.
“In the case of disseminated metastatic disease, the prognosis is poor, and the mortality rate is high,” explains Dr. Gupta. “At least 17% of the patients are in the high-risk category and they have only about 11-40% 5-year survival. So, this is clearly an unmet need and a clinical challenge.”
NSC668394 was identified in a screen of a large National Cancer Institute library of small molecules. In explaining the rationale behind her study, Dr. Gupta noted that previous positive results of NSC668394 in osteosarcoma and their own findings in adult large cell lymphoma prompted the investigation of this agent in RMS. “Osteosarcoma and RMS are two important sarcomas in the pediatric world and may share common pathogenic mechanisms,” she says. Previous research by other groups has shown that suppression of ezrin expression in highly metastatic RMS cell lines reduces their metastatic potential, whereas boosting ezrin expression in poorly metastatic RMS cell lines leads to higher rates of metastasis.
Advertisement
This latest study assessed the antitumor activity of NSC668394 using an in vitro and an in vivo preclinical model. Four cell lines were used in in vitro experiments, representing the major histological subtypes and fusion status of RMS.
“Our results have shown that you can induce dephosphorylation and inactivation of ERM proteins using this agent in tissue culture,” said Dr. Gupta. “We also showed that it induces a loss of survival of RMS cells by inducing caspase-3-mediated apoptosis.” The induction of cleaved caspase-3 following treatment with NSC668394 was observed in all RMS cell lines tested, with maximal increase observed at 48 hours.
Dr. Gupta’s team also used two different mouse models — the subcutaneous xenograft model and the orthotopic (intramuscular) xenograft model — to test the agent in vivo. “We noticed a significant decrease in the tumor burden of mice treated with NSC668394 in both models,” said Dr. Gupta.
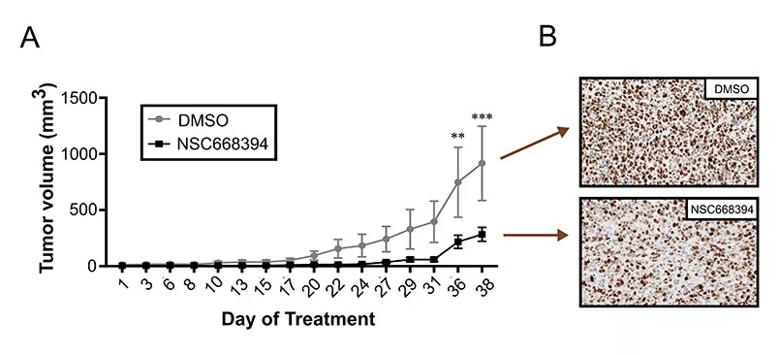
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7874c838-71c0-48af-861a-8e268f835d0b/805x-Inset-ERM-Novel-Therapy-1_jpg)
Quantitative image analysis of NSC668394-treated tumors sections (n = 5) showed decreased Ki67 (p < 0.01) when compared to DMSO-treated tumor sections. Image originally published in: Proudfit A, Bhunia N, Pore D, Parker Y, Lindner D, Gupta N. Pharmacologic inhibition of ezrin-radixin-moesin phosphorylation is a novel therapeutic strategy in rhabdomyosarcoma. Sarcoma. 2020. https://doi.org/10.1155/2020/9010496
Since ezrin is an intracellular protein with important functions in healthy cells, including signal transduction, adhesion and cell migration, Dr. Gupta’s team was initially concerned about potential undesirable toxicity and tolerability of NSC668394.
Advertisement
“We looked at the grooming behavior of the mice, and their weight, and noticed that there were no significant effects on these two parameters and no apparent toxicity in mice treated with this molecule,” says Dr. Gupta. “So, the agent was tolerated well in our studies.”
While there were no apparent adverse effects in tested animals, Dr. Gupta notes that additional toxicity profiling studies are still needed to confirm that the agent does not accumulate in individual organs.
“We would like to perform a full profiling of this agent’s toxicity in vivo,” she says and adds that it is also important to work in parallel on identifying new agents with increased oral bioavailability. “Our first goal is to develop newer, more potent and orally bioavailable ERM inhibitors. We are collaborating on this with the therapeutic discovery core in the Lerner Research Institute, so that we can perform structural modeling and use medicinal chemistry approaches to screen new molecules.”
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity

Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time

Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology