Rationale and status reports on mechanistically varied strategies
BaBy Manmeet S. Ahluwalia, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Glioblastoma (GBM), the most common and lethal primary malignant brain tumor, still has limited effective treatment options. Current standard therapies — including surgery, radiation and conventional chemotherapies — have not been able to extend survival much beyond 15 to 18 months.
Physicians and basic scientists from Cleveland Clinic’s Rose Ella Burkhardt Brain Tumor and Neuro-Oncology Center and Lerner Research Institute are closely collaborating on efforts across multiple fronts to find a breakthrough to combat this daunting disease. Their multidisciplinary partnership has contributed to Cleveland Clinic’s status as home to one of the largest and most active brain tumor clinical trial programs in the U.S. (Figure).
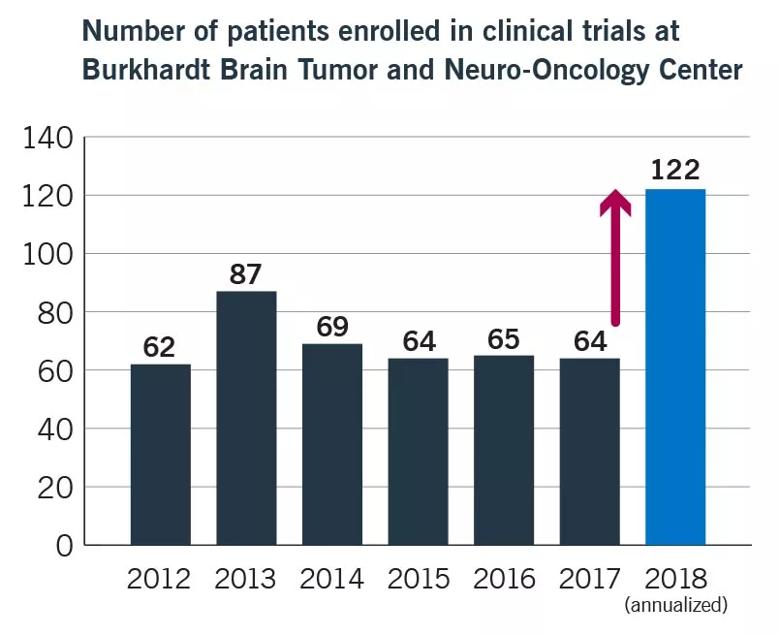
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/51591567-a869-41ea-9e39-838cf9e36957/18-NEU-4915-Ahluwalia-Pathways-Graph_jpg)
Figure. Cleveland Clinic’s brain tumor clinical trials program is one of the nation’s largest, with expected enrollment of 122 patients in 2018.
Our institution’s large, international patient population and deep, broad scientific and clinical resources promote the rapid translation of promising basic research findings to clinical trials. Below are four representative examples of ongoing GBM clinical trials that stem from translational research projects and highlight the bench-to-bedside efforts of our multidisciplinary teams.
GBM confounds conventional therapies by suppressing the host immune system and bouncing back from the guns fired at it. Medical oncologist David Peereboom, MD, and stem cell biologist Justin Lathia, PhD, both with the Rose Ella Burkhardt Brain Tumor and Neuro-Oncology Center, are leading efforts to understand the mechanism of immunosuppression and develop innovative therapies to address it. Rather than targeting tumor cells — the usual focus of GBM therapeutics — their strategy is to reverse the immunosuppressed microenvironment of GBM to reduce tumor growth.
Advertisement
Their research is focused on human myeloid-derived suppressor cells (MDSCs), which have potent immunosuppressive qualities. The work is built on our findings that MDSCs are elevated in the blood of patients with GBM and also present close to self-renewing cancer stem cells in the tumors themselves. It is believed that the tumor secretes factors that promote migration of host MDSCs to the brain, where they are activated by cancer stem cells, resulting in blocking of beneficial host antitumor immune responses. The result: promotion of GBM growth and metastasis.
Enter capecitabine — an oral analogue of 5-fluorouracil (5-FU) — which is cytotoxic to MDSCs. Currently used to treat colorectal cancer, metastatic breast cancer and (off-label) several other cancers, capecitabine offers a possible novel approach to treating GBM.
In the current phase 0/1 clinical trial for which Dr. Peereboom is principal investigator, patients with recurrent GBM and planned tumor resection are treated with a seven-day cycle of capecitabine, then surgery, then capecitabine again, combined with bevacizumab. Bevacizumab is a standard therapy for GBM; although it blocks angiogenesis and slows tumor growth, it does not extend survival.
This proof-of-concept study will help determine whether MDSC suppression is feasible by evaluating the change in concentration of circulating MDSCs following treatment. The concentration of MDSCs in the resected tumor and blood will also be evaluated, as will the concentration of T-regulatory cells, using new technology such as mass cytometry time of flight, which allows simultaneous assessment of more than 30 parameters. This approach, just reported in a large-scale analysis of brain tumor patients (Alban et al., JCI Insight. 2018 Nov 2 [Epub ahead of print]), will allow the team to further pinpoint key changes in the immune system associated with favorable response. Progression-free survival and adverse effects will be assessed as well.
Advertisement
Dr. Peereboom will present preliminary results of this trial at the Society for NeuroOncology’s annual meeting in November 2018. Early evidence is encouraging, and his team expects to further advance this strategy.
Ibrutinib, a small-molecule compound recently approved by FDA to treat various forms of lymphoma and leukemia, is being evaluated by Shideng Bao, PhD, in a phase 1 study for its application to GBM. Dr. Bao is a researcher in Cleveland Clinic’s Department of Stem Cell Biology and Regenerative Medicine.
A major challenge in GBM treatment has been the inability to effectively target the glioma stem cell (GSC) population that gives rise to tumor recurrence. Earlier work by Dr. Bao’s group found that GSCs have high levels of BMX (bone marrow and X-linked nonreceptor tyrosine kinase). BMX activates signal transducers and promoters of transcription 3 (STAT3), which resist radiation therapy and enable GSCs to replicate, spread and promote tumor growth. Ibrutinib specifically disrupts the BMX-mediated STAT3 activity (see Shi et al., Sci Trans Med. 2018; doi: 10.1126/scitranslmed.aah6816).
In a preclinical mouse model of GBM and cultured human GBM cells, Dr. Bao’s team found that ibrutinib suppressed GSC-driven tumor growth and potently induced GSC death. It was significantly more effective in slowing tumor growth than temozolomide, the current standard-of-care chemotherapy for GBM. Average survival increased significantly in preclinical models. His team’s work has also demonstrated that ibrutinib has excellent blood-brain barrier penetration.
Advertisement
The current phase 1 clinical trial is testing various ibrutinib dosages for safety and efficacy in patients with newly diagnosed methylated or unmethylated MGMT GBM. The study is combining ibrutinib with radiation in the patients with unmethylated MGMT promoter. Patients with methylated MGMT GBM will have temozolomide added to their treatment regimen.
Patient accrual is ongoing and expected to be completed in the summer of 2019.
Another investigation targeting GSCs uses ruxolitinib, a drug currently used to treat myelofibrosis and polycythemia vera. A Janus kinase (JAK) inhibitor (specifically of JAK1 and JAK2), ruxolitinib targets the JAK-STAT pathway, which has been implicated in GBM as a promoter of tumor cell survival, growth and invasion. Levels of JAK 1/2 and STAT3 are increased in GBM tissues.
Our group and others have found that inhibiting JAK2 reduces survival and proliferation of glioma cells in vitro and that JAK2/STAT3 inhibition slows disease progression in animal models of GBM. We have since initiated a phase 1 trial testing efficacy, safety and tolerability of ruxolitinib combined with radiation and temozolomide for newly diagnosed grade 3 gliomas and GBM. The study’s combination of ruxolitinib and radiation is anticipated to facilitate breakdown of the blood-brain barrier and delivery of ruxolitinib to the tumor in the unmethylated MGMT promoter arm. Patients with methylated MGMT promoter will receive various doses of ruxolitinib, temozolomide and radiation.
Delivering hyperthermia shortly before radiation therapy is likely to sensitize cancer stem cells to radiation. That’s the premise of an ongoing phase 0 clinical trial being led by Jennifer Yu, MD, PhD, of Cleveland Clinic’s Department of Radiation Oncology and Department of Stem Cell Biology and Regenerative Medicine.
Advertisement
The study builds on a publication by Dr. Yu’s team showing that hyperthermia sensitizes glioma stem-like cells to radiation by inhibiting AKT signaling in a preclinical orthotopic model of human GBM (Man et al., Cancer Res. 2015;75:1760-1769). The team then confirmed these findings in a mouse model of GBM, showing that animals that received both hyperthermia and radiation survived longer than those receiving radiation alone.
The present phase 0 trial is evaluating the effect of shortening the time between laser interstitial thermal therapy (LITT) and subsequent adjuvant chemotherapy and radiation in patients with GBM. The hope is that a shortened interval will allow radiation oncologists to take advantage of the biological properties of LITT, such as sensitizing cancer stem cells to radiation, priming the immune system and opening up the blood-brain barrier.
These four innovative translational studies are the direct result of basic research that yielded greater understanding of cellular mechanisms of GBM. Our hope is that targeted cellular and immunotherapy approaches will soon make headway against this disease that has so far confounded conventional therapeutic approaches.
Dr. Ahluwalia, a neuro-oncologist in the Rose Ella Burkhardt Brain Tumor and Neuro-Oncology Center, is principal investigator of the ibrutinib and ruxolitinib studies.
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity
Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time
Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology