And substudy reveals good outcomes with PASCAL system in patients with complex mitral valve anatomy
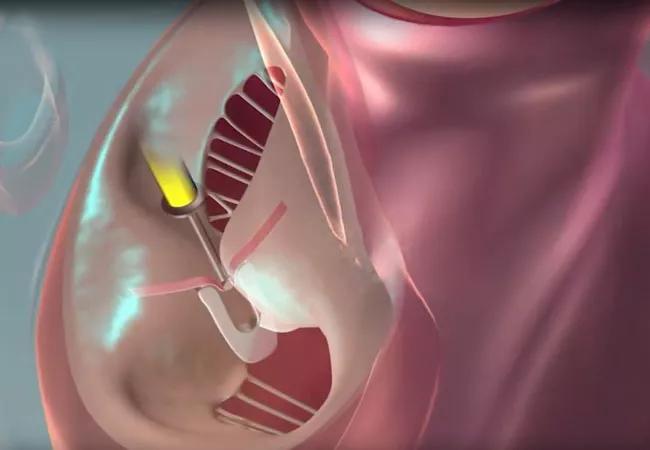
After one year, intervention with the PASCAL transcatheter valve repair system demonstrated noninferiority to intervention with the more established MitraClip™ system for mitral valve transcatheter edge-to-edge repair (M-TEER). So conclude investigators with the CLASP IID randomized clinical trial comparing the two M-TEER systems in 300 patients with severe degenerative mitral regurgitation (MR) at prohibitive risk for mitral valve surgery. The one-year data update from the prospective trial was presented at the 2023 Transcatheter Cardiovascular Therapeutics (TCT) meeting and simultaneously published online in JACC: Cardiovascular Interventions.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“At one year, we detected no significant differences between PASCAL and MitraClip in terms of complications, mortality and other clinical outcomes,” says study co-author Samir Kapadia MD, Chair of Cardiovascular Medicine at Cleveland Clinic and principal investigator of CLASP IID’s Cleveland Clinic site. “The results support PASCAL as a good therapy option for high-risk patients.”
The international multicenter CLASP IID trial (NCT03706833) is the first to directly compare the PASCAL and MitraClip systems for significant symptomatic degenerative MR in patients at prohibitive surgical risk. Primary results in an initial cohort of 180 patients were published last year in JACC: Cardiovascular Interventions (2022;15[24]:2523-2536). At 30 days, the PASCAL system met safety and efficacy noninferiority endpoints, demonstrated high survival and low complication rates, and was associated with significant and sustained MR reduction at six months.
Based in part on these data, the PASCAL system received FDA approval in September 2022. The new report includes primary and secondary endpoint outcomes for the enlarged cohort out to one year.
The CLASP IID update was based on 300 patients at 54 sites in the U.S., Canada and Europe. All had significant degenerative MR (3+/4+), with most patients classified as 4+, and all were ineligible for surgery. Patients were randomized 2:1 to PASCAL (n = 204) or MitraClip (n = 96).
During the study period, different generations of both systems were used as design advances became available. The latest-generation MitraClip G4 system was used in 68.4% of the MitraClip arm; the latest PASCAL Precision system was used in 21.1% of the PASCAL arm, with most cases treated with the first-generation system.
Advertisement
Noninferiority of PASCAL for the primary endpoints — safety (30-day composite major adverse events) and efficacy (6-month MR ≤ 2+) — persisted for the entire cohort. Major adverse events at 30 days occurred in 4.6% of the PASCAL group and 5.4% of the MitraClip group.
One-year outcomes included the following, with all meeting noninferiority criteria between the two systems:
In both groups, reduction in MR severity was associated with significant reductions in left ventricular volumes at one year, indicating favorable reverse left ventricular modeling. Additionally, significant and comparable gains were achieved in the two groups at one year in New York Heart Association functional classification and in two quality-of-life measures, the Kansas City Cardiomyopathy Questionnaire score and EuorQol-5 Dimention-5 Level visual analog score. Improvement from baseline in 6-minute walk distance was not statistically significant but was comparable between the two groups.
Mean procedural time was longer in the PASCAL group than in the MitraClip group (88 vs. 81 minutes; P = 0.014). Mean transmitral gradients were similar between the groups (2.5 ± 1.06 mmHg in the PASCAL group vs. 2.3 ± 1.06 mm Hg in the MitraClip group; P = 0.167), as was mean mitral valve area (5.9 ± 1.30 cm2 vs. 6.2 ± 1.56 cm2, respectively; P = 0.512). At one year, there were no single-leaflet device attachments (SLDAs) in the MitraClip group, whereas there were four SLDAs (2.0%) in the PASCAL group — three of which occurred within 30 days and one within six months of the procedure.
Advertisement
“Greater procedural time and more SLDAs can be due to early experience with PASCAL,” notes Dr. Kapadia. “Interestingly, the gradients and mitral valve areas were not different between the two devices; this is somewhat unexpected, because the PASCAL system has a spacer that is intended to reduce gradient across the mitral valve and increase the mitral valve area.”
The CLASP IID study also included a group of 98 patients with complex mitral valve anatomy who were ineligible for MitraClip based on its use instructions but who were deemed suitable for and underwent M-TEER with the PASCAL system. Results were separately presented at the 2023 TCT meeting and simultaneously published online in JACC: Cardiovascular Interventions.
Major one-year outcomes in this subgroup were as follows:
“PASCAL demonstrated good safety and efficacy in this very high-risk group of patients” Dr. Kapadia notes.
“Outcomes at one year in CLASP IID are very reassuring, especially considering this very frail population who are not surgical candidates,” Dr. Kapadia concludes.
“These recent data show us that the two TEER devices are similar in safety and efficacy, and our extensive experience in percutaneous mitral valve repair at Cleveland Clinic has given us proficiency in use of both,” adds Amar Krishnaswamy, MD, Section Head of Invasive and Interventional Cardiology. “Over time, we are likely to better understand whether there are specific anatomies that are better treated by one device or the other, which will allow us to further refine and optimize care for our patients.”
Advertisement
The CLASP IID investigators will continue to follow patients annually through five years.
CLASP IID was funded by Edwards Lifesciences, which markets the PASCAL device.
Advertisement
Advertisement

How Cleveland Clinic is using and testing TMVR systems and approaches

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

NIH-funded comparative trial will complete enrollment soon

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients

Multimodal evaluations reveal more anatomic details to inform treatment

Two surgeons share insights on weighing considerations across the lifespan