A perplexing presentation underscores the value of broadly collaborative care
A man in his 50s contacted Cleveland Clinic from out of state through its virtual second opinion program after suffering recurrent thromboembolic phenomena following his second surgical aortic valve replacement. He was connected with cardiothoracic surgeon Shinya Unai, MD.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
After Dr. Unai reviewed the patient’s records and spoke with him on the phone, the patient decided to pursue treatment at Cleveland Clinic. While arrangements were being made, he suffered several episodes of transient neurological symptoms. He was admitted to a local hospital and treated for presumed seizures. His local cardiologist was concerned that he had an underlying issue with his prosthetic valve and subsequently contacted Dr. Unai to say he thought the patient needed surgery urgently. Dr. Unai initiated a transfer to Cleveland Clinic, and the patient arrived the next day.
Upon transfer to Cleveland Clinic, the patient was admitted under the care of cardiac imaging specialists Rhonda Miyasaka, MD, and Christine Jellis, MD, PhD, in the Department of Cardiovascular Medicine, in collaboration with Dr. Unai. Consults to neurology, hematology and infectious diseases were also expedited. Advanced cardiac imaging was integral to rapid evaluation of the patient on arrival. A 3D transesophageal echocardiogram (TEE) (Figure 1) showed a large mobile echodensity attached to the aortic valve bioprosthesis, which was prolapsing from the valve backwards into the left ventricular outflow tract. Given this patient’s history, lack of infective symptoms and negative blood cultures, differential diagnosis pointed to prosthetic valve thrombus as the most likely explanation.
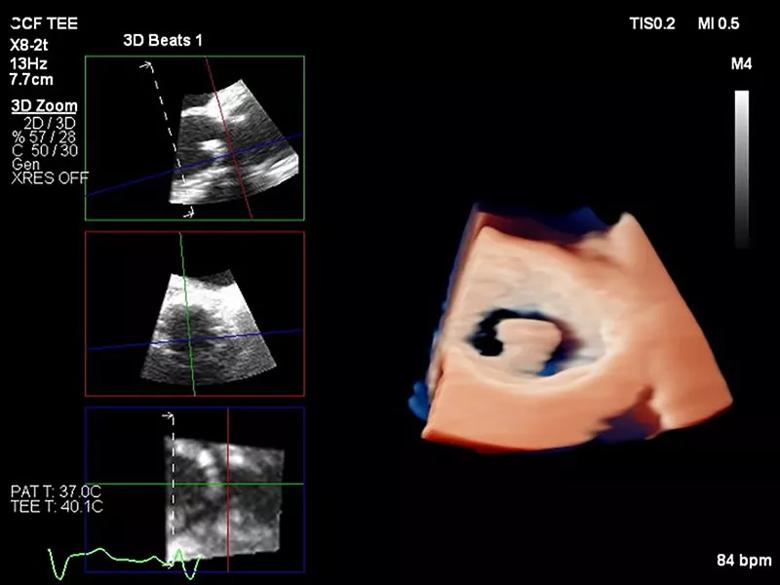
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/306c29cb-0aea-4624-a097-4475e05b3bb8/21-NEU-2527887-CQD-Inset-1-800x600-1_jpg)
Figure 1. 3D transesophageal echocardiography with use of multiplanar reconstruction to demonstrate the large presumed thrombus adherent to the prosthetic aortic valve prolapsing backwards into the left ventricular outflow tract.
Advertisement
Given the concern for embolic risk, plans for valve surgery with Dr. Unai were expedited, with all consulting teams partnering to determine the safety and timing of surgery, given the patient’s recurrent short-lived but disabling episodes of left-sided facial droop and left-sided arm and leg numbness and weakness, which were consistent with transient ischemic attacks.
During this time, the patient experienced a further neurological episode, and the stroke team was activated via Cleveland Clinic’s internal acute stroke emergency (2CLOT) process. Symptoms resolved completely within minutes, so no acute intervention was performed. However, as a precaution, the patient was transferred to the neurological ICU for close monitoring. He continued to have recurrent short episodes of significant left facial droop and weakness as previously described.
Subsequent urgent MRI and MR angiography of the brain and carotid arteries raised suspicion of a subocclusive calcified thrombus at the origin of the superior M2 middle cerebral artery branch with reduced blood flow to the corresponding vascular distribution and multiple small acute lacunar and cortical infarcts. Although the patient was outside the conventional therapeutic window for mechanical thrombectomy, after extensive discussion with the patient and care teams, the decision was made to pursue a cerebral angiogram. The study confirmed a subocclusive lesion at the origin of the superior M2 division of the right middle cerebral artery.
Vascular and interventional neurologist Gabor Toth, MD, undertook a procedure to extract the material causing the blockage of the middle cerebral artery branch by combining a stent retriever device with a self-expanding basket and a suction aspiration catheter. Visual inspection of the extracted lesion revealed calcified material, which was later confirmed on pathological examination. The debris was suspected to have originated from the patient’s degenerating prosthetic aortic valve (Figure 2).
Advertisement
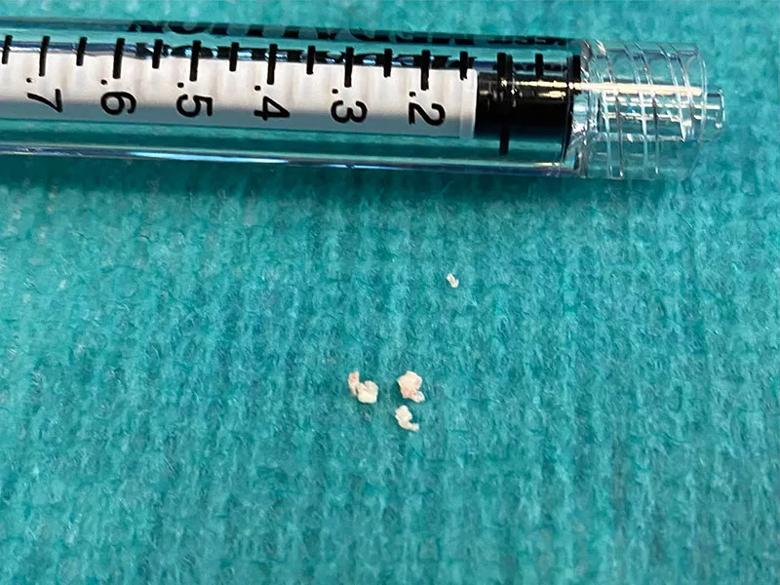
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9589d60a-0aaf-4203-b558-5ec8fe2b5daa/21-NEU-2527887-CQD-Inset-2-800x600-1_jpg)
Figure 2. Photograph of calcifications removed from the patient’s right middle cerebral artery.
Angiography immediately after thrombectomy revealed patent vessel with no residual blockage or other filling defects downstream. After uneventful postprocedural monitoring, the patient was transferred to a regular nursing floor. Two days later, having had no further neurological episodes, he was cleared for the initially planned redo cardiac surgery.
Two days after that, Dr. Unai replaced the patient’s thrombosed prosthetic aortic valve and root with a homograft. Given the patient’s two prior episodes of valve thrombus and the possibility of infection/endocarditis, use of a homograft — which is least thrombogenic and resistant to infection — was thought to be ideal. The patient’s intraoperative and postoperative courses were uneventful, and he was discharged home seven days later.
Pathology confirmed that the valve lesion was thrombus (Figure 3), without evidence for underlying infective endocarditis. The patient was diagnosed with a hypercoagulable state of unknown etiology. Given his recurrent arterial and venous thrombi, he will remain on lifelong anticoagulation and will be followed by hematology.
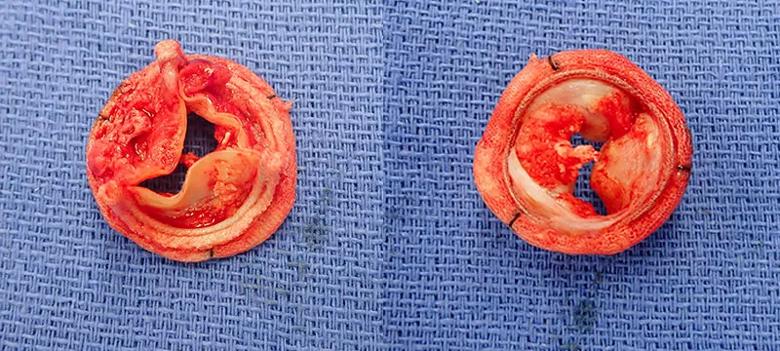
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/786d50c0-132d-4232-9428-605a88f69e47/21-NEU-2527887-CQD-Inset-3-800x360-1_jpg)
Figure 3. Photographs of the explanted valve. Large amounts of thrombus were attached to the valve, which was partially calcified.
Although this patient initially self-referred for valve replacement, identifying and treating the underlying cause of his neurological symptoms became a priority.
Advertisement
“If we had taken him to cardiac surgery initially with a subocclusive vascular blockage in the brain, even mild blood pressure fluctuations could have resulted in a major ischemic stroke,” notes Dr. Toth. “The high dose of anticoagulants required for surgery could have also caused a secondary brain hemorrhage.”
The unusual nature of these fast-resolving neurological symptoms was perplexing and required close collaboration by a multidisciplinary team to rule out endocarditis and other etiologies for repeated valve thrombi.
Hematology staff were asked to evaluate the patient to determine whether he had an underlying clotting disorder that had not been identified. Their input helped inform the type of valve to be implanted and the type of anticoagulation used afterward.
“Dr. Unai’s management exemplified a team approach,” says Dr. Jellis. “Pulling in cardiology, neurology, hematology and infectious diseases optimized the patient’s clinical stability and made a complex situation more like routine management.”
Once it was determined that cerebral flow was likely being restricted by embolized calcified valvular debris, a new set of challenges arose.
“Our tools are mostly designed to remove clots,” Dr. Toth points out. “In contrast, we have no specific tools for retrieving debris.” His response was to tailor existing tools safely and efficiently to resolve the patient’s unique problem.
“We decided to use a combination of devices with the highest chance of successfully removing the debris in one piece,” he says. “Fortunately, it worked.”
Advertisement
Dr. Unai chose to replace the patient’s aortic valve with a homograft from a cadaver. “It is extremely rare for homografts to develop thrombi,” he notes.
Endocarditis with root or annular abscess is the primary indication for an aortic valve homograft. In addition, homografts provide superior hemodynamics appropriate for a young and active patient like the one in this case, Dr. Unai explains.
Because Cleveland Clinic has a large experience with homograft implants, Dr. Unai was confident that this patient’s third valve operation could be performed safely. Indeed, the patient was extubated on the day of surgery, stayed in the cardiac ICU just two days and was discharged home in seven days.
“The key to success in unusual cases like this is to get the patient to a tertiary or quaternary care center where multiple specialists can put their heads together and come up with options that provide the best chance of uncovering and solving the problem,” Dr. Toth concludes.
Advertisement

How Cleveland Clinic is using and testing TMVR systems and approaches

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients

Multimodal evaluations reveal more anatomic details to inform treatment

Insights on ex vivo lung perfusion, dual-organ transplant, cardiac comorbidities and more