Surgery is typically the only option for the most severe cases, but a minimally invasive procedure is reducing morbidity and recovery time for patients
Tracheobronchomalacia (TBM) has become an overarching term that incorporates many diseases in the airway, which in their sort of essence are a form of dynamic airway occlusion. There are several causes of the condition, including congenital anomalies, inflammatory disease, damage to the cartilage from tracheostomy or surgery. Still, the primary symptom is either a laxity of the posterior membrane of the airway or a problem with the integrity of the cartilages, which leads to dynamic airway problems.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
For patients with severe TBM, the standard surgery, tracheoplasty, involves stabilizing the central airway via a thoracotomy, but it comes with significant morbidity due to the major chest incision involved. A new surgical approach — a minimally-invasive robotic procedure — has shown reduced morbidity, a shorter recovery time for patients and significant improvement among patients.
“Surgery is really only an option for patients who are over 90% occlusion of the airway, so these are the most severe patients,” explains Thomas Gildea, MD. “But because TBM can be a symptom of so many other diseases and conditions, the difficulty of it is teasing out how much are airway-derived symptoms or how much is related to an underlying condition that just needs to be better managed. The diagnosis process to determine this is quite involved.”
Dr. Gildea notes that the first part of the process is to meet with the patient to discuss the symptoms they are experiencing with their illness. Next, the care team will go down parallel pathways of figuring out whether an underlying condition could lead to these symptoms.
“We’ll look at the airway using a dynamic CT or an inspiratory and expiratory CT,” explains Dr. Gildea. “There’s a specific protocol where we’ll do CT scans as the patient inhales and exhales to see how much the airway changes. If we see an airway abnormality there or it looks suspicious, then we would move on to a bronchoscopy. So, a bronchoscopy in that context becomes the gold standard to really understand the extent and severity of the airway collapse.”
Advertisement
He continues, “A bronchoscopy is done under a specific protocol with minimal sedation and a small scope. We do a series of forced exhalations during the bronchoscopy to see if we can identify exactly where and how severe the abnormality of the airway is. The goal here is to try to quantify what percentage of the airway is obstructed and see how far it extends down into the airways. When we’re in there, we typically go get cultures and biopsies of the airways to see if there are any other abnormalities that are contributing. We can also apply CPAP and see if we can modify the airway collapse with what amounts to pneumatic stenting.”
Following the radiographic workup and the bronchoscopy, the care team classifies the condition. One method, the FEMOS classification tool looks at Functional status, Extent, Morphology, Origin and Severity. This provides a measurement of how much airway hypermobility is at the given segments. “This helps us get a sense of how severe and significant the condition is and whether they need to go on to advanced therapies, i.e., a stent trial or tracheobronchoplasty. We typically only use the stent trial if there’s some sense that the patient would be a surgical candidate,” says Dr. Gildea.
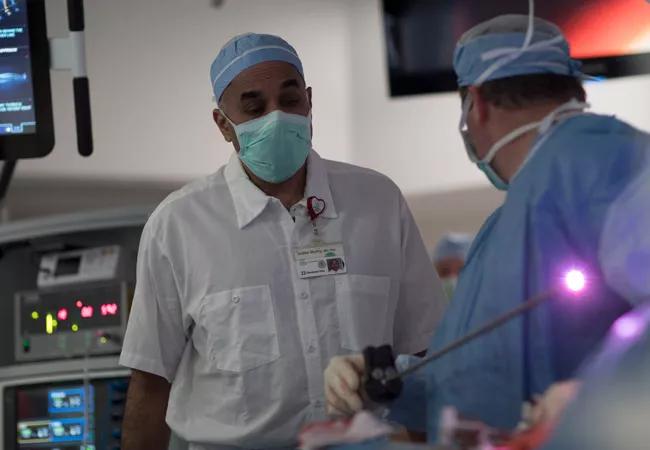
The original surgical approach for treating TBM involved a thoracotomy through a large incision. While the concept of the original surgery remains — using sutures and mesh to pull the cartilages together to provide stability — this is now done robotically. The floppy airway is stitched to a mesh structure that stiffens up and prevents the cartilage from spreading out.
Advertisement
“Because of the large incision in the chest, there was significant morbidity involved,” says Dr. Gildea. “There were also several complications associated with having big chest surgery. Plus, this surgery is often done in patients with severe lung disease, which adds to the challenge. Now, it’s a robotic procedure, and it’s done with small minimally invasive surgery in ports.
“Our approach is to divide the operation into two parts: first, we want to expose the trachea and bilateral bronchi, and the second part is the tracheobronchoplasty itself,” says Sudish Murthy, MD, Section Head of Thoracic Surgery and the Surgical Director of the Center of Major Airway Disease at Cleveland Clinic.
“Patients are usually discharged on the third-day post-op, which is much sooner than they would be with the original surgery,” says Dr. Murthy. “Additionally, because this approach is minimally invasive, there is much less associated morbidity than the original surgery. Patients still report some associated pain with the new approach, but again, it’s much less than the original surgery where we were opening the chest.”
Dr. Murthy notes that they will typically see the patient at three months to repeat a battery of testing, including pulmonary function testing and walk testing. Imaging is also repeated, and the patient will undergo a dynamic CT scan again to confirm that the surgery did in fact reduce the hypermobility of the airway radiographically.
A bronchoscopy is also typically performed on these patients to further ensure no other complications associated with the surgery or any endobronchial problems that may have arisen. Patients continue to be monitored every three months for the first year after surgery, and after that, it depends on the disease.
Advertisement
“Most people seem to reach peak within three to six weeks or so after they recover, any residual pain is resolved,” explains Dr. Murthy. “But we’ve had some patients who feel dramatically better right away.”
“Although we’ve seen great results with this surgical approach, there are still some long-term questions that need to be answered,” says Dr. Gildea. “There are some reports that some patients start to exhibit failure over time, so there may be some questions about what the surgery’s long-term durability is. Although the stent trial is done as part of our standard of care at Cleveland Clinic, some places don’t do a stent trial, and so it would be interesting to know if that is really a critical piece of the puzzle.”
He continues, “Figuring out how to better measure outcomes is also important. Anecdotally, patients report feeling much better after the surgery with notable improvement in various quality of life measures, but interestingly, sometimes the pulmonary function doesn’t get better or in fact gets a little worse after surgery. So, we need to determine if different measures may be more appropriate to specific patient populations because we have people who report feeling so much better, but we’re not able to demonstrate consistent objective measure in all cases.”
Advertisement
Advertisement

Case study illustrates the potential of a dual-subspecialist approach
New tools and protocols to improve care

Endoscopic balloon dilation during pregnancy helps optimize outcomes
Cleveland Clinic pulmonologists share a framework for how to implement effective clinical protocols to standardize evaluation and management of complex acute respiratory distress syndrome

A public health tragedy with persistent pathophysiological and therapeutic challenges

Recent breakthroughs have brought attention to a previously overlooked condition

Findings show profound muscle loss variance between men and women
For the first time, risk is shown after accounting for underlying contributions of pulmonary disease