Hypoxic induction of vasorin regulates Notch1 turnover
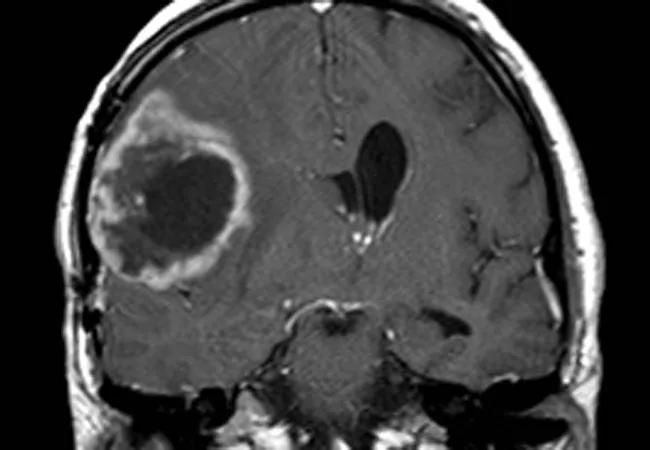
Much of the research of Jennifer Yu, MD, PhD, explores the molecular mechanisms of brain cancer. Navigating the complex interactions among molecules in a cell that can trigger particular processes — perhaps turning a gene on or off or nurturing particular cell types — isn’t always easy to describe succinctly, so she likes to use analogies whenever possible.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
For instance, when Dr. Yu, Department of Stem Cell Biology and Regenerative Medicine, Lerner Research Institute, and Department of Radiation Oncology, Cleveland Clinic Cancer Center, describes her work on cancer stem cells (CSCs), she evokes the image of a queen termite. Unless the queen is eliminated, the colony will survive; the same is true of CSCs. Without targeting them, recurrences can occur.
CSCs are elusive and difficult to destroy, especially CSCs that reside in hypoxic areas of tumors where radiation and chemotherapy are less effective. With this in mind, Dr. Yu and colleagues recently undertook an investigation to learn more about the molecular mechanisms of CSCs in hypoxic regions of glioma tumors.
The team first studied human glioma samples from a tissue database to identify candidate proteins that might be involved in this pathway. They found that a protein called vasorin — known to be induced in hypoxic settings — was abundant in patients with aggressive brain cancers who had poorer survival.
Next they studied vasorin in cell culture to determine how it affects glioblastoma progression. Under normal conditions, an adaptor protein binds to and inhibits the pro-cancer Notch pathway (important for cell proliferation, differentiation and survival). They found that in a hypoxic environment, however, the abnormally abundant vasorin instead binds to and switches on the Notch signaling pathway in glioblastoma stem cells, leading to unchecked tumor growth.
“I think with vasorin we found an Achilles’ heel for the glioma stem cells in these hypoxic areas,” Dr. Yu says.
Advertisement
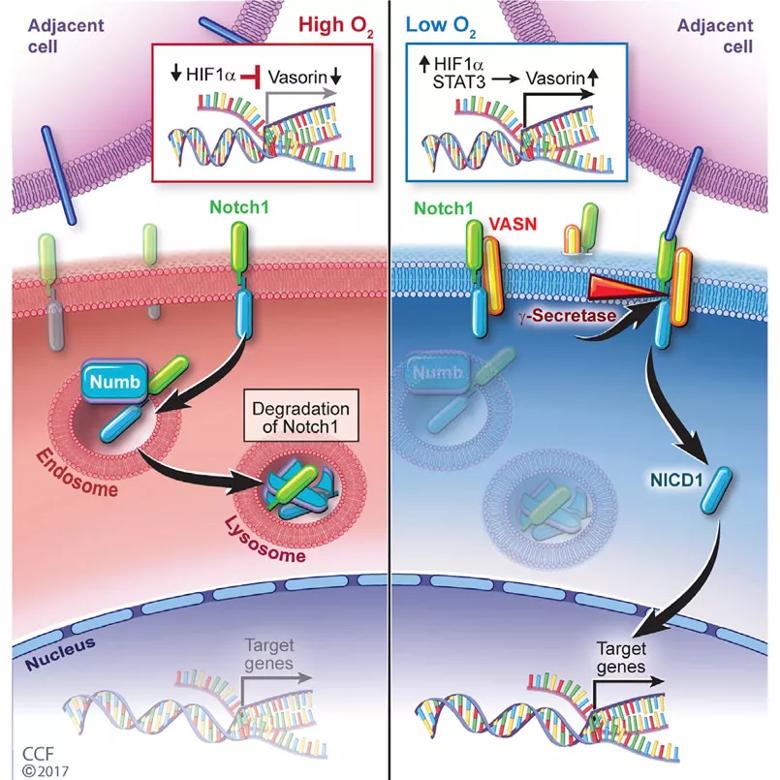
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/c1160482-af52-484f-8f06-f7ca25fdfa18/vasorin_805x805_jpg)
Republished with permission from Elsevier.
The research, which was support by a $1.7 million NIH grant and published in Cell Stem Cell, is one of many projects that Dr. Yu has undertaken in her quest to better understand brain cancer and methods for treating it. In addition to molecular investigations, she also has projects looking at radiomics and radiation necrosis in brain cancer patients and the use of hyperthermia to treat brain tumors.
“About one-third of cancer patients will develop brain metastases,” Dr. Yu says. “The aim of my lab is to understand how these metastases adapt to their environment at the molecular level and how we can target them effectively.”
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity

Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time

Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology