JACC State-of-the-Art Review details current knowledge and new developments
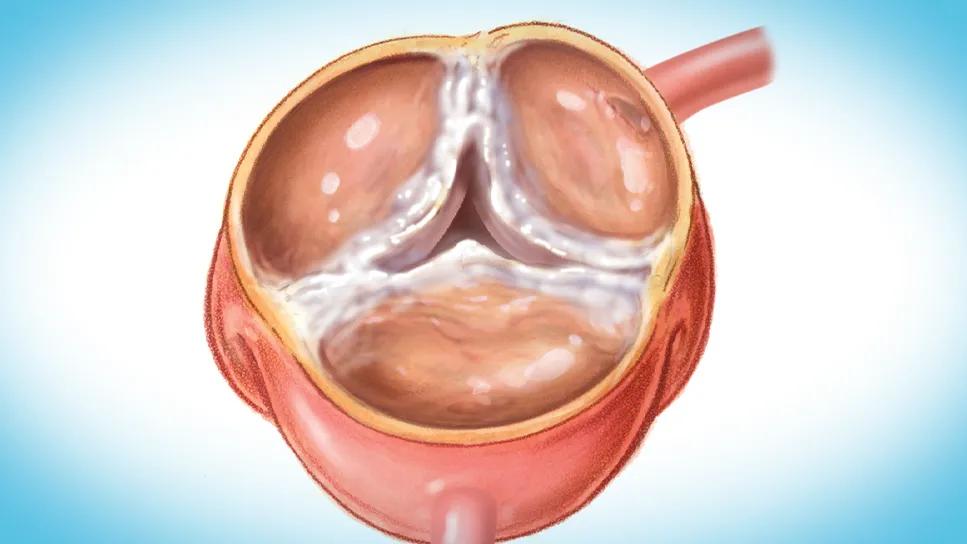
Greater understanding of the pathogenesis of calcific aortic valve stenosis (CAVS) — leading to development of new pharmaceuticals and earlier intervention to stave off progression — is an important goal for better managing this common debilitating condition. So contend two leading CAVS experts in a recently published State-of-the-Art Review in the Journal of the American College of Cardiology (JACC) (2025;86[9]:659-672).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“We are starting to appreciate that CAVS is not simply a passive degenerative process associated with aging but that it involves active mechanisms that may be amenable to intervention,” says Milind Desai, MD, MBA, Vice-Chair, Heart, Vascular and Thoracic Institute, Cleveland Clinic, who co-authored the review with Eugene Braunwald, MD, of Brigham and Women’s Hospital in Boston. “We believe we can improve on the current commonly employed strategy of watchful waiting until patients become eligible for aortic valve replacement.”
The review details the epidemiology of CAVS, current understanding of its underlying mechanisms, its typical course and the latest thinking on how to improve assessment of disease severity and management. Potential new pharmaceutical agents and imaging strategies are also discussed.
Multiple overlapping processes appear to contribute to the initiation and propagation of aortic valve leaflet calcification. They include oxidative stress, lipoprotein deposition leading to chronic inflammation, induction of osteogenic signaling cascades and calcium deposition in damaged tissue. Such processes provide potential targets for therapy to slow disease progression.
As CAVS progresses and the aortic valve gradient increases, left ventricular hypertrophy advances and cardiac output declines. Patients develop reduced exercise capacity and increased likelihood of experiencing a cardiovascular event. Without aortic valve replacement (AVR), a patient with severe aortic stenosis with angina, syncope or congestive heart failure has a median survival of only two to five years.
Advertisement
Traditionally, markers of CAVS progression (increased transvalvular gradients, reduced aortic valve areas) are used to guide decisions on the need for AVR. However, the review authors argue, waiting for severe aortic stenosis to develop misses a potential opportunity to intervene at an earlier stage when patients may benefit.
Recent studies indicate that the phase of silent progression of valve calcification entails increased risk of morbidity and mortality. As a result, earlier elective AVR — before adverse effects on left ventricular structure and increased risk of heart failure development — improves outcomes compared with conservative care.
However, early AVR has potential drawbacks. “Because AVR entails risk and sometimes requires reintervention down the road, we need novel pharmacotherapies to fill the gap for patients with mild to moderate CAVS,” says Dr. Desai.
With the emerging possibility of new ways to intervene before severe CAVS develops (see below), there’s a mounting need for novel tools and biomarkers to detect CAVS earlier, better assess disease severity, and monitor progression and therapy response.
Aortic valve calcification, quantifiable with noncontrast CT, accurately identifies severity of aortic stenosis and predicts death adjusted for age, sex, velocity and aortic valve area. Separate thresholds for severity have been determined for men and women, as the latter tend to have a different pattern of aortic stenosis, with less calcification than men for a given hemodynamic severity. A similar more severe pattern is found in patients with previous chest radiation for mediastinal or lung disease. Quantified aortic valve calcification was recently found to be associated with risk of mortality in patients with either low-flow or paradoxical low-flow aortic stenosis.
Advertisement
Another possible approach to identifying early disease is detection of microcalcification activity with sodium fluoride (18F-NaF) positron emission tomography. It could be especially useful for research into new pharmacotherapies.
At this point, no approved medications exist to slow CAVS progression. Drugs to reduce cholesterol, including statins and ezetimibe, and vitamin K2 to reduce calcium deposition have demonstrated no benefit and are not recommended by current guidelines for the treatment of CAVS.
“Our evolving understanding of the pathogenesis of CAVS indicates that underlying mechanisms are different from those involved with atherosclerosis,” Dr. Desai explains.
“We may be entering an era of new medical therapies that can slow progression of CAVS, along with new ways to detect early disease and monitor interventions,” he continues. “These developments offer the hope of dramatically changing how we currently manage this disease.”
The review profiles pharmacotherapies that have been studied to date for use in slowing CAVS progression. One of the most promising appears to be ataciguat, a novel once-daily drug targeting the soluble guanylate cyclase inhibitor pathway, which acts on signaling cascades implicated in oxidative stress and inflammation of valvular cells. In a small phase 2 clinical trial, ataciguat showed benefit for patients with moderate CAVS in slowing progression of aortic valve calcification as well as other changes in valvular and ventricular dysfunction.
Advertisement
In the wake of those findings, Katalyst-AV (NCT07001800) — a phase 3, double-blind, placebo-controlled trial of ataciguat — is now recruiting patients with moderate aortic stenosis. Dr. Desai is global principal investigator of the multicenter international trial, which is expected to enroll 1,400 patients and be completed by 2030. Cleveland Clinic is an enrolling site, and the Cleveland Clinic Coordinating Center for Clinical Research (C5Research) is managing the trial’s operation.
“With the aging population, aortic stenosis is increasing in prevalence, and no drugs are currently approved to slow the progression of this disease,” says Steven Nissen, MD, Chief Academic Officer of Cleveland Clinic’s Heart, Vascular and Thoracic Institute. “The development of a drug to slow the progression of aortic stenosis would have tremendous public health consequences for the millions of patients at risk.”
Advertisement
Advertisement

Age and other factors figure into the choice among SAVR, TAVR, Ross, Ozaki and more

Optimally timed valve replacement depends on an expert approach to nuanced presentations
Large longitudinal study supports earlier intervention over clinical surveillance

Series of 145 patients characterizes scope of presentations, interventions and outcomes

New Cleveland Clinic data challenge traditional size thresholds for surgical intervention

Study finds comparable midterm safety outcomes, suggesting anatomy and surgeon preference should drive choice

New review distills insights and best practices from a high-volume center

When to recommend aortic root surgery in Marfan and other syndromes