Technique may lay groundwork for personalized decision-making in procedural intervention
Determining which patients with ischemic cardiomyopathy (ICM) could benefit from surgical intervention is difficult due to the complexity and variability of individual patients’ cardiac pathology and the difference between randomized trial results and expected clinical outcomes.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Now, Cleveland Clinic researchers have published an advanced analysis of clinical and imaging data from hundreds of ICM patients (Circ Cardiovasc Imaging. Epub 2024 Apr 16) that yields insights that may improve future therapeutic management.
The researchers’ analytic approach is known as cardiac magnetic resonance (CMR)-enriched phenomapping because it incorporates patients’ medical imaging to help identify ICM phenotypes.
The analysis, driven by machine learning, revealed three distinct phenotypic clusters of patients whose CMR characteristics and post-revascularization survival outcomes differ significantly. It is the first large CMR-enriched phenomapping study to predict the varying survival benefits of surgical interventions in patients with ICM.
Though confirmatory studies are needed, the researchers say their findings could pave the way for personalized risk prediction and better decision-making in ICM treatment.
“CMR-enriched phenomapping could provide the platform to enable precision medicine, giving us an augmented ability to select the best treatment management for patients with complex disease,” says Cleveland Clinic cardiologist Deborah Kwon, MD, Director of Cardiac MRI and the study’s lead author.
Attempts to predict whether and how ICM patients will respond to therapeutic intervention — specifically, to surgical revascularization and treatment of secondary mitral regurgitation (MR) — are complicated by the range of patients’ pathophysiologies, including infarct size and the resulting extent and features of cardiac remodeling.
Advertisement
“Patients with ischemic cardiomyopathy are very complex,” Dr. Kwon says. “They have many competing risks and it’s often difficult to tease out the components that are interrelated.
“Also, there are a lot of studies that are counter to what we expect from a clinical practice perspective,” she says. “Many of us use viability testing, for instance, to assess what part of the myocardium is alive, to then decide whether the patient should be referred for bypass surgery or if we should open the vessel. Theoretically, if the vessel is closed or narrowed and the underlying part of the heart is alive, you should open the pathway to restore blood supply. But recent studies suggest that’s not helpful to predict outcomes or select which patients should be revascularized.”
Dr. Kwon hypothesized that inclusion of heterogenous ICM phenotypes may have confounded the results in randomized trials and that elucidating these distinct phenotypes might explain the differential clinical outcomes.
CMR captures important cardiac tissue characterization as well as structural, volumetric and functional measures, thus enabling comprehensive phenotyping. However, because of limited access to CMR, existing risk scores for surgical revascularization and mitral valve intervention do not incorporate CMR data.
Dr. Kwon and her colleagues turned to machine learning and CMR-enriched unsupervised phenomapping to determine whether clinically meaningful subgroups of ICM patients existed. “We thought this approach could help explain some of the conflicting evidence in the literature,” she says.
Advertisement
Phenomapping is a type of machine learning applied to patient data to find mathematical relationships and use them to cluster patients into distinct phenotypic groups with mutually exclusive diagnostic traits. To avoid bias, unsupervised phenomapping is conducted without information in the dataset about intervention types or outcomes, and without explicit instructions on how to relate data points.
Dr. Kwon and her colleagues applied unsupervised phenomapping to clinical, demographic and CMR imaging data from 787 patients with advanced ICM treated at Cleveland Clinic between 2001 and 2017. Of those patients, 380 (48.3%) were revascularized and 157 (19.9%) underwent surgical mitral valve intervention. Four hundred forty-six patients died, 25 eventually received cardiac transplants and 19 were implanted with a left ventricular assist device.
Unsupervised phenomapping clustered the observational study’s patients into three distinct phenogroups. Subsequent analysis showed the clustering was driven primarily by distinct differences in patients’ CMR features — particularly myocardial infarct size and the degree of adverse cardiac remodeling — and, to a lesser extent, by clinical variables.
Phenogroup 1 (Figure 1) was defined by intermediate infarct size and limited remodeling; phenogroup 2 (Figure 2) by small infarct size, intermediate remodeling, multi-vessel coronary artery disease and significant secondary MR; and phenogroup 3 (Figure 3) by large infarct size, advanced remodeling and more significant secondary MR.
Advertisement
Video content: This video is available to watch online.
View video online (https://cdnapisec.kaltura.com/p/2207941/sp/220794100/playManifest/entryId/1_36en91v7/flavorId/1_5f3sgelj/format/url/protocol/https/a.mp4)
Video content: This video is available to watch online.
View video online (https://cdnapisec.kaltura.com/p/2207941/sp/220794100/playManifest/entryId/1_euc7l4eg/flavorId/1_5f3sgelj/format/url/protocol/https/a.mp4)
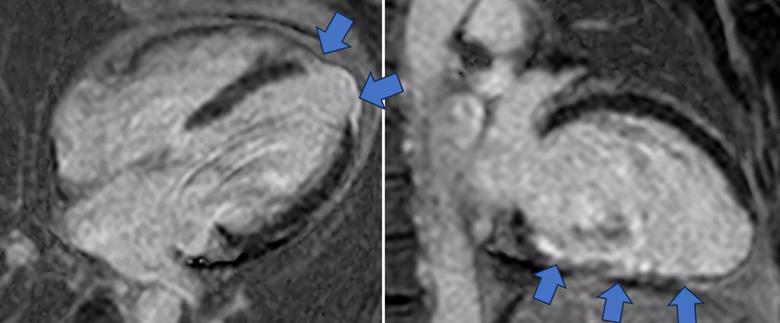
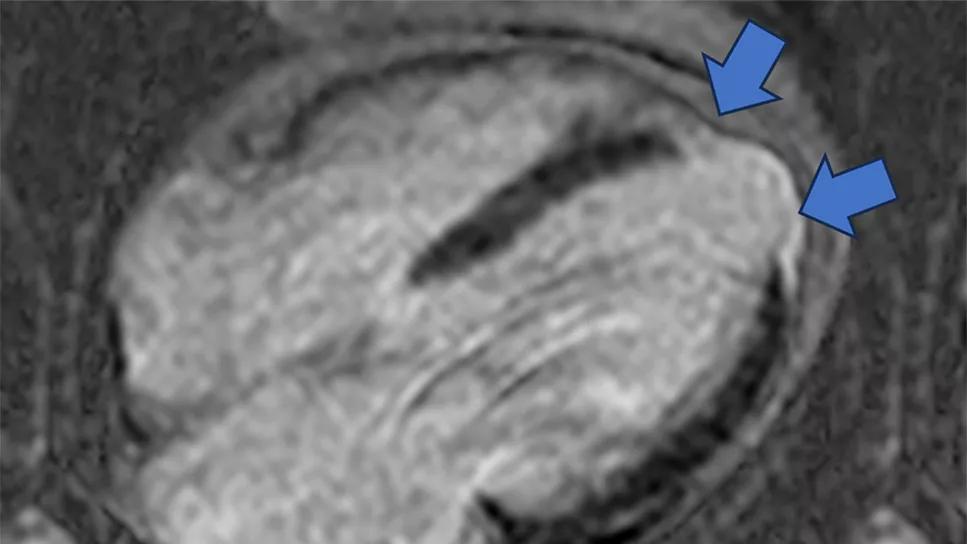
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/79465cb2-a249-4ac7-aa79-48e03df9f48c/cine-MRI-for-phenoogroup-1)
Figure 1. Representative cine SSFP (steady-state free precession) images (top rows) and late gadolinium enhancement images (bottom row) from a phenogroup 1 patient. Blue arrows indicate areas of late gadolinium enhancement that identify myocardial scarring from prior myocardial infarction.
Video content: This video is available to watch online.
View video online (https://cdnapisec.kaltura.com/p/2207941/sp/220794100/playManifest/entryId/1_m8cp5uc8/flavorId/1_5f3sgelj/format/url/protocol/https/a.mp4)
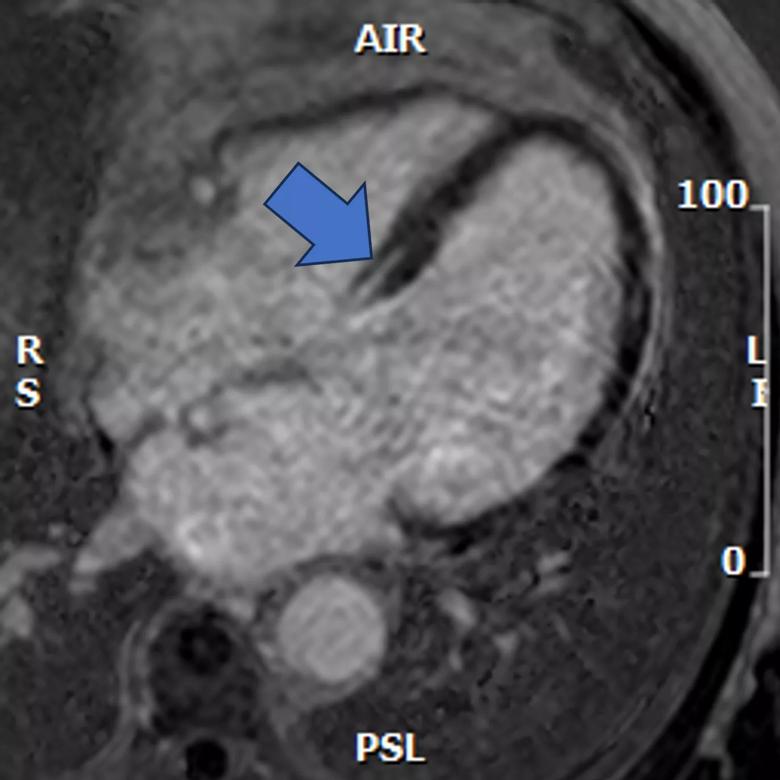
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/765c7d40-26fc-4aae-95ca-fcd2eb350935/cine-MRI-for-phenoogroup-2)
Figure 2. Representative cine SSFP image (top) and late gadolinium enhancement image (bottom) from a phenogroup 2 patient. The blue arrow indicates late gadolinium enhancement/myocardial scarring in a nonischemic pattern.
Multivariable analysis showed that patients in phenogroup 3 received significant survival benefit (P = .0081) from revascularization and surgical mitral valve intervention, but patients in phenogroups 1 and 2 did not.
Video content: This video is available to watch online.
View video online (https://cdnapisec.kaltura.com/p/2207941/sp/220794100/playManifest/entryId/1_8tte20rc/flavorId/1_5f3sgelj/format/url/protocol/https/a.mp4)
Video content: This video is available to watch online.
View video online (https://cdnapisec.kaltura.com/p/2207941/sp/220794100/playManifest/entryId/1_ll62kvbx/flavorId/1_5f3sgelj/format/url/protocol/https/a.mp4)
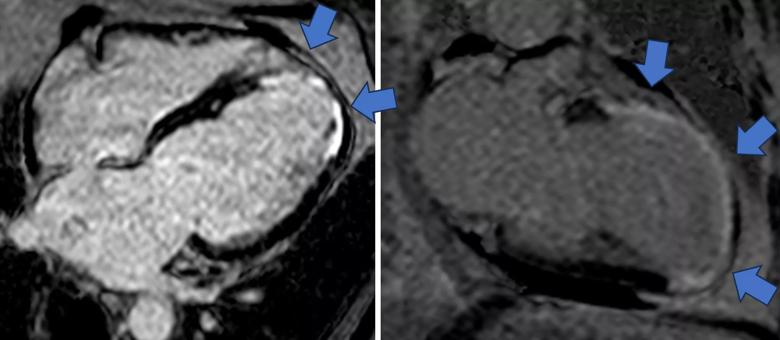
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/134b034e-04d4-4ba1-b1d2-a2ea6aa1656e/cine-MRI-for-phenoogroup-3)
Figure 3. Representative cine SSFP images (top rows) and late gadolinium enhancement images (bottom row) from a phenogroup 3 patient. Blue arrows indicate areas of late gadolinium enhancement that identify myocardial scarring from prior myocardial infarction.
Initially, it may seem counterintuitive that phenogroup 3 patients — i.e., those with the most extensive cardiac scarring, nonviable tissue and adverse remodeling — would derive the greatest benefit from revascularization and mitral intervention.
“I thought the patients who derive the most survival benefit would be the patients with preserved myocardial substrate, but it actually was the other way around,” Dr. Kwon says. “The patients who were at the highest risk were actually the patients who had the most to gain.”
She and her co-authors hypothesize that surgery may improve survival for these high-risk patients by decreasing ischemic burden, improving myocardial function, reducing adverse remodeling in viable tissue and stabilizing arrhythmic substrate.
Also seemingly counterintuitive is that patients in phenogroup 2 — who had the smallest infarct size and were the most likely to undergo post-CMR revascularization — would not see significant survival benefit from the surgery.
“Patients with so much viable tissue were always thought to be the patients who have the most to gain,’” Dr. Kwon explains. “And patients with large amounts of hibernating myocardium are certainly likely to benefit from revascularization. However, concomitant underlying nonischemic cardiomyopathy may be present in a significant number of patients who have extensive myocardial dysfunction but minimal myocardial scarring. And these patients with concomitant underlying nonischemic cardiomyopathy may not necessarily benefit from revascularization and may benefit more from optimized guideline-directed medical therapy.”
Advertisement
Similarly, Dr. Kwon offers a potential explanation for why phenogroup 1 patients, with intermediate infarct size and limited adverse remodeling, don’t derive significant survival benefit from revascularization.
“In those patients, the myocardial reserve seemed to be more robust than in group 3,” she says. “So, one could theorize that those patients’ hearts had already found a way to compensate such that revascularization may not offer survival benefit over guideline-directed medical therapy.”
Essential future steps for the researchers are to better understand the rationale and biological underpinnings for the phenomapping patient clusters, assess the clinical potential of CMR-enriched phenomapping, and determine whether it can impact ICM treatment outcomes.
“It’s incumbent on us as a medical community to figure out whether this clustering is meaningful, what are the biological explanations for why these groups are different from each other, and then how this approach can be applied,” Dr. Kwon says.
She hopes to conduct a randomized clinical trial evaluating CMR-enriched phenomapping’s utility in guiding patient selection to optimize outcomes.
“CMR-enriched phenomapping is a good tool to point us in certain directions,” Dr. Kwon says. “What’s needed is more exploration to understand why these findings are as they are and to validate them in a prospective randomized controlled trial. If the findings are positive, then we need to determine how best to implement this technique into clinical practice.”
Her research and clinical colleagues agree. “CMR is a powerful imaging technology capable of characterizing the heart at the tissue level,” says study co-author Christopher Nguyen, PhD, Director of the Cardiovascular Innovation Research Center and Director of MRI Research at Cleveland Clinic. “Combining it with radiomics can give us further insights in phenotyping our patients. This study marks an important step toward meaningful precision medicine using novel radiomic CMR features for our patients with ischemic cardiomyopathy. The phenomapping presented in this study demonstrates a data-driven way to hone in on exactly which feature is important.”
“Many patients with ischemic cardiomyopathy and left ventricular dysfunction who have severe mitral regurgitation can benefit from transcatheter edge-to-edge repair (TEER),” adds co-author Amar Krishnaswamy, MD, Section Head of Invasive and Interventional Cardiology. “However, it can be difficult to understand which patients would or would not benefit from TEER, and it becomes more difficult as left ventricular function declines. With the caveat that we don’t yet have a robust clinical outcomes dataset, it appears — at least based on this early-stage trial — that CMR-based phenomapping is an excellent step in the right direction to help the heart team with patient selection.”
Advertisement

Medical and surgical perspectives on current and emerging uses of ECMO and Impella

Least-invasive open-heart AVR option to date yielded rapid recovery in all cases

Launch of the tool promises to reshape quality assessment across the specialty
Lead dwell time and manufacturer emerge as independent predictors of success in registry study
The case for a thoughtful approach to CTO and minimally invasive options for CABG

How the tool could help physicians alter management in real time
Preoperative Impella 5.5 placement can provide a critical safety net for high-risk patients

Check out our latest volume and outcomes data in these key areas