Logistic feasibility supported for treating obstructive HCM under the REMS program
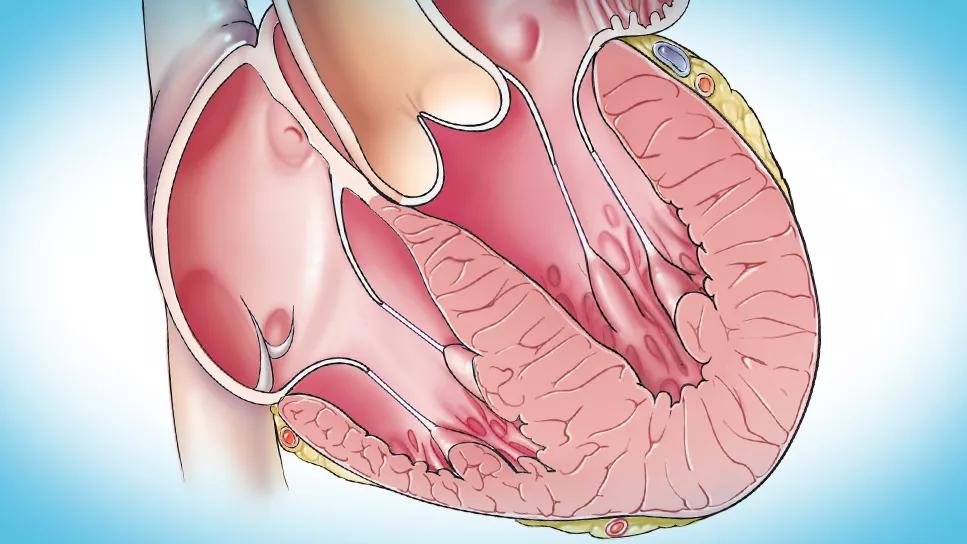
Mavacamten, only available through the FDA Risk Evaluation and Mitigation Strategy (REMS) program because of the potential for development of heart failure, demonstrated safety and efficacy in a real-world prospective observational study of 150 patients with symptomatic obstructive hypertrophic cardiomyopathy (HCM). The study was published by Cleveland Clinic researchers in Progress in Cardiovascular Diseases (Epub 2024 Feb 13).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Consistent with clinical trial findings, most patients in this report realized functional gains, improved left ventricular outflow tract (LVOT) gradients and reduced eligibility for septal reduction therapy (SRT) at 12 weeks. Just 2% of patients had temporary drug interruption for left ventricular ejection fraction (LVEF) of less than 50%, and no patients developed LVEF of less than 30% or heart failure requiring hospitalization — results that compared favorably with those from clinical trials of mavacamten, the researchers noted.
“Notably, the logistics of managing mavacamten under the REMS program were successfully addressed, indicating feasibility in general practice,” says the study’s corresponding author, Milind Desai, MD, MBA, Director of the Hypertrophic Cardiomyopathy Center at Cleveland Clinic. “In addition, the low rate of mavacamten interruption in this and other real-world reports indicates that echocardiographic monitoring may not need to be as frequent as suggested by clinical trial data.”
Septal myectomy, the leading form of SRT, is considered the gold standard for treating obstructive HCM. But because of the surgery’s inherent risk and complexity, it should only be performed at experienced tertiary care surgical centers, limiting this option to relatively few patients.
Mavacamten was FDA-approved for symptomatic obstructive HCM in April 2022 after results from phase 3 clinical trials demonstrated improvements in LVOT gradient, symptoms and physical function, as well as reduced SRT eligibility. The novel cardiac myosin inhibitor was the first medication approved for this indication.
Advertisement
However, because the drug also reduces LVEF — increasing risk of heart failure — its use is restricted to availability only through the REMS program. The program requires monitoring for heart failure with periodic echocardiograms, and interruption of the drug for LVEF less than 50% or heart failure symptoms. In addition, because of potentially dangerous interactions with certain cytochrome P450 inhibitors, the REMS also requires screening for drug interactions each time mavacamten is dispensed.
Because of the potential risk of mavacamten use and uncertainty about how well providers can meet the REMS restrictions, obtaining real-world data is especially important. “Our current study reports on a larger and more geographically varied population with a longer follow-up than other published real-world studies to date,” Dr. Desai notes.
The study cohort consisted of the first 150 adults who were started on mavacamten 5 mg at Cleveland Clinic facilities in Ohio and Florida for symptomatic obstructive HCM. The patients had a mean age of 65 years, 53% were women, 83% were taking beta-blockers and 61% were in New York Heart Association (NYHA) class III. Patients with LVEF less than 55% at baseline were excluded.
NYHA class, LVEF and LVOT gradients were determined at baseline and at 4, 8 and 12 weeks. Mavacamten dosage was titrated per assessments of symptoms and echocardiography.
Key findings were as follows:
Advertisement
“The improvements we observed mirror those seen in clinical trials,” says Dr. Desai. “But several important differences were also observed that provide important lessons to clinical practice.” He highlights the following:
“Using these practices, it’s possible that echocardiographic monitoring need not be so stringent as recommended based on the clinical trials,” adds study co-author Zoran Popovic, MD, PhD, a cardiologist in Cleveland Clinic’s Section of Cardiovascular Imaging. “Further long-term data to determine if symptomatic improvement and reduction of the need for SRT are sustained will also help inform the use of this important new drug.”
Advertisement
Advertisement

Emerging evidence suggests a patient-specific approach

Multidisciplinary care for patients with immune-related adverse events

Cleveland Clinic will offer rapid, pinpoint airborne transport of medications and other medical items
Additional analyses of the two trials presented at 2023 ESC Congress

Looking at the real-world impact and the future pipeline of targeted therapies

End-of-treatment VALOR-HCM analyses reassure on use in women, suggest disease-modifying potential

Study reveals key differences between antibiotics, but treatment decisions should still consider patient factors

Cardiac imaging substudy is the latest paper originating from the VANISH trial