Avoidance of septal reduction therapy continues while LVEF dysfunction remains infrequent
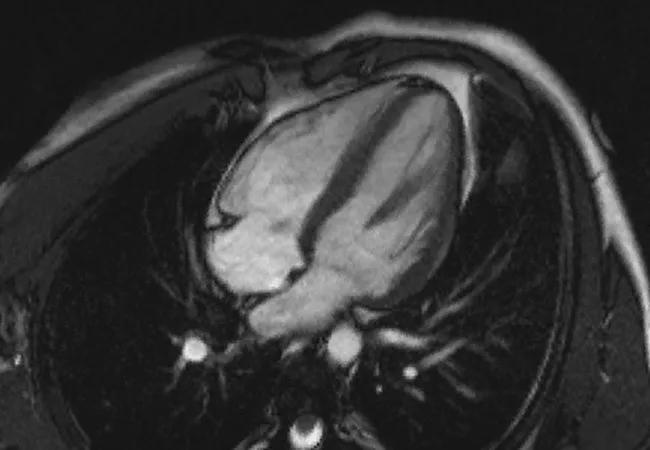
More than two years of therapy with the oral reversible cardiac myosin inhibitor mavacamten continues to be associated with reduced guideline eligibility for septal reduction therapy (SRT) as well as sustained improvements in clinical and cardiac imaging measures in the vast majority of patients with severely symptomatic obstructive hypertrophic cardiomyopathy (HCM).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
These end-of-study findings of the VALOR-HCM trial (NCT04349072), with data through 128 weeks of therapy, were recently presented at the American Heart Association Scientific Sessions 2024 and simultaneously published in Circulation.
“VALOR-HCM supports extended use of mavacamten as a viable long-term therapeutic option for patients with symptomatic obstructive HCM who were referred for SRT,” says the study’s first and corresponding author, Milind Desai, MD, MBA, Director of Cleveland Clinic’s Hypertrophic Cardiomyopathy Center. “The drug was well tolerated, and sustained freedom from SRT was observed at 128 weeks, with nearly 90% of patients remaining on long-term mavacamten.”
HCM is a disease of hypercontractility of heart muscle that leads to dyspnea and reduced exercise capacity due to dynamic left ventricular outflow tract (LVOT) obstruction and mitral regurgitation. Patients are at increased risk for heart failure, atrial fibrillation and sudden death. Beta-blockers, calcium channel blockers and disopyramide may help with symptoms but do not change disease progression.
The most effective treatment for HCM is SRT, an invasive surgery that improves quality of life and survival. Due to its difficulty, the procedure is best conducted at specialized centers with experienced surgeons, limiting access for most patients.
VALOR-HCM, a phase 3, double-blind, placebo-controlled trial, was designed to evaluate treatment with mavacamten in patients with severely symptomatic obstructive HCM who met recommendations for SRT. Based on early results demonstrating reduced need for SRT and favorable effects on quality of life, diastolic function and cardiac remodeling, the drug was approved for treatment of obstructive HCM in the United States and other countries.
Advertisement
Although mavacamten is now recommended in guidelines as an option for managing patients with this condition, longer-term safety and efficacy data have been lacking.
Conducted at 19 U.S. sites, the trial evaluated 108 SRT-eligible patients (56 initially randomized to mavacamten and 52 initially randomized to placebo and transitioned to mavacamten after 16 weeks). Patients (mean age of 60.3 years, 50% female) were severely symptomatic (94% New York Heart Association [NYHA] class III or IV) at baseline and on maximally tolerated oral therapy. Left ventricular ejection fraction (LVEF) had to be at least 60% and resting oxygen saturation at least 90% to qualify for the study.
The principal endpoint was the percentage of patients who underwent SRT or remained guideline-eligible for it.
At trial’s end — with 128 weeks of mavacamten exposure (112 weeks for the group originally randomized to placebo) — the following key results were found among the 108 patients:
Advertisement
In safety analysis, 15 patients (13.9%) developed an LVEF of less than 50%. Of these, 12 patients continued mavacamten at a lower dose (all were asymptomatic) and three patients had permanent mavacamten discontinuation; two of the latter three patients developed LVEF ≤ 30%, one of whom had sudden cardiac death. New-onset atrial fibrillation occurred in 11 patients (10.2%), translating to a rate of 4.55/100 patient-years.
The drug was well tolerated by most patients, and the majority (88%) opted to transition to commercially available mavacamten following the trial’s end.
At baseline, 34% of patients were taking at least two oral medications for HCM versus just 13% when taking mavacamten.
The investigators note that these findings are in addition to prior data demonstrating improved long-term quality of life and favorable cardiac remodeling with mavacamten, including improvement in left atrial and left ventricular strain.
Careful monitoring is required to avoid excessive reduction of left ventricular systolic function. Echocardiography was used for dose titration and safety assessment in this study, as opposed to plasma mavacamten concentration testing.
While atrial fibrillation occurred in 10% of participants during the study, atrial fibrillation has a reported incidence of 34% (23% de novo) among patients following myectomy. “HCM patients are at high risk of atrial fibrillation, so it would be difficult to demonstrate a causal relationship between any therapy and atrial fibrillation onset,” Dr. Desai notes. He adds, however, that careful attention to LVEF should be paid during mavacamten therapy in patients with a prior history of atrial fibrillation.
Advertisement
“There is limited availability of high-volume SRT centers. In this context, this study shows that a medical therapy represents a useful therapeutic option by providing sufficient improvement so that patients may no longer need SRT,” Dr. Desai concludes.
“These data give us further reassurance in using mavacamten with close monitoring of patients, which is promoted through the REMS program that the FDA has required for the medication,” adds Cleveland Clinic cardiologist Maran Thamilarasan, MD, who was not involved in the study. “The results give us a very good option for patients who remain symptomatic with LVOT obstruction despite standard therapy. We are hopeful for potential longer-term benefits as well.”
He continues: “There will still be a valuable role for surgical septal reduction in a subset of patients with obstructive HCM and in those who elect to proceed with this surgical solution. Our institution is fortunate to have a team with the surgical expertise to offer this with excellent long-term results.”
Cardiac surgeon Nicholas Smedira, MD, MBA, leads that team as Surgical Director of the Hypertrophic Cardiomyopathy Center. “These results show that we now have a drug that can be used once patients are evaluated by a multidisciplinary team specializing in the treatment of obstructive HCM,” says Dr. Smedira, who was a co-investigator with the VALOR-HCM trial. “Treatment decisions during these evaluations should be made on a case-by-case basis, and we welcome having another option to offer patients.”
Advertisement
The VALOR-HCM trial was funded by Bristol Myers Squibb, parent company of MyoKardia, the developer of mavacamten. Dr. Desai reports that he has received consulting fees from Bristol Myers Squibb.
Advertisement

End-of-treatment VALOR-HCM analyses reassure on use in women, suggest disease-modifying potential

Cardiac imaging substudy is the latest paper originating from the VANISH trial

Vigilance for symptom emergence matters, a large 20-year analysis reveals

Phase 3 ODYSSEY-HCM trial of mavacamten leaves lingering questions about potential broader use

5% of flagged ECGs in real-world study were from patients with previously undiagnosed HCM

High composite score in myectomy specimens signals worse prognosis
Few patients report left ventricular dysfunction or heart failure after one year

New risk score pools factors that may predict adverse outcomes in the uncommon phenotype