Protocol for cardiac surgical backup saves the day after SVC injury
By Gosta Pettersson, MD, PhD; Stephanie Mick, MD; Oussama Wazni, MD; Alok Dash, MD; and Andrew Bauer, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
A 56-year-old woman presented to the emergency department with “vibrations” from her implantable cardioverter defibrillator (ICD). Such vibrations are a feature of ICDs designed to alert patients to changes in device function that may require a physician’s attention. She had a history of left mastectomy and non-ischemic cardiomyopathy secondary to adriamycin and radiation therapy for breast cancer.
Preoperative chest X-ray (Figure 1) showed three leads. Because of her left-sided breast cancer and disrupted lymphatic system, the ICD device had been implanted on the right side.
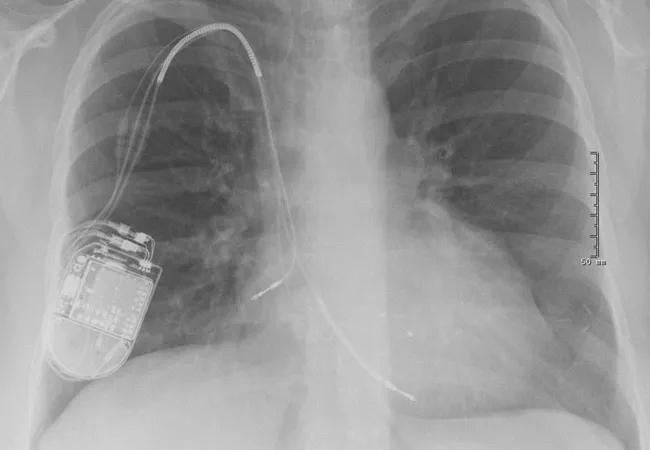
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/5ac34f39-54e5-4e32-9d90-e33c9858c976/18-HRT-117-Big-Rescues-CQD-Hero_jpg)
Figure 1. Preoperative chest X-ray showing right atrial, right ventricular and coronary sinus leads.
Her CRT defibrillator generator had been changed five years earlier, with a retained coronary sinus lead from which she did not benefit. The ICD lead had been recalled because of failures, so the patient was being followed, but extraction had been delayed because of patient anxiety about the procedure.
At the current presentation, interrogation revealed high right ventricular shocking impedance. Ejection fraction was 30 percent, but she was otherwise asymptomatic. The decision was made to transvenously extract and reimplant an ICD lead in a hybrid operating room.
During the extraction procedure, the patient developed severe acute hypotension with tamponade. Echocardiogram showed a new pericardial effusion (Figure 2). She quickly became asystolic, and CPR was initiated. An emergency median sternotomy was performed while another team member inflated the rescue balloon that was in place.
Advertisement
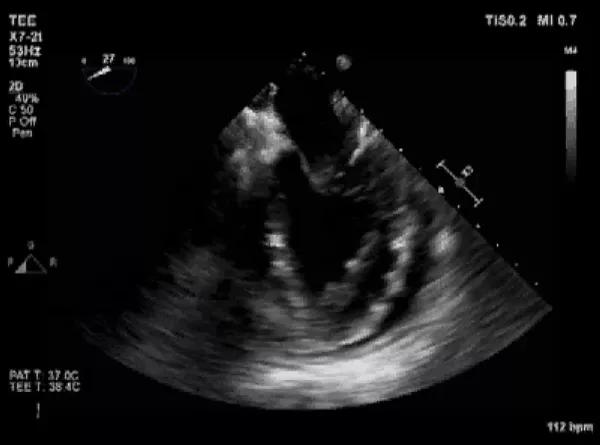
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/4367217f-e199-4b82-b8df-6e0d402b8a40/18-HRT-117-Big-Rescues-CQD-Inset-2_jpg)
Figure 2. Echocardiogram from the time of lead extraction showing new pericardial effusion.
After the pericardium was open, the tamponade was relieved. Initially, the site of bleeding was unclear, but was soon localized to the superior vena cava (SVC). Digital pressure to the SVC temporarily abated the bleeding. At this point, echocardiography showed new tricuspid regurgitation (Figure 3).
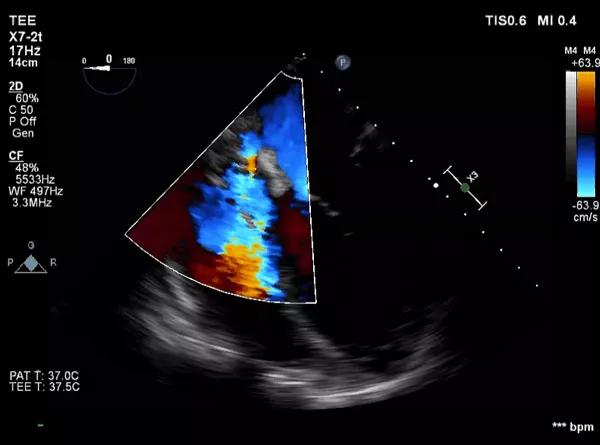
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/c73ae7c6-2894-45c8-aefa-22e112d757a2/18-HRT-117-Big-Rescues-CQD-Inset-3_jpg)
Figure 3. Echocardiogram showing tricuspid regurgitation with fluid compressing the right atrium and fluid around the left ventricle.
Further inspection revealed a 4-cm posterior SVC wall tear from below the innominate vein and up to the subclavian and internal jugular vein confluence. Figure 4 depicts the normal venous anatomy.
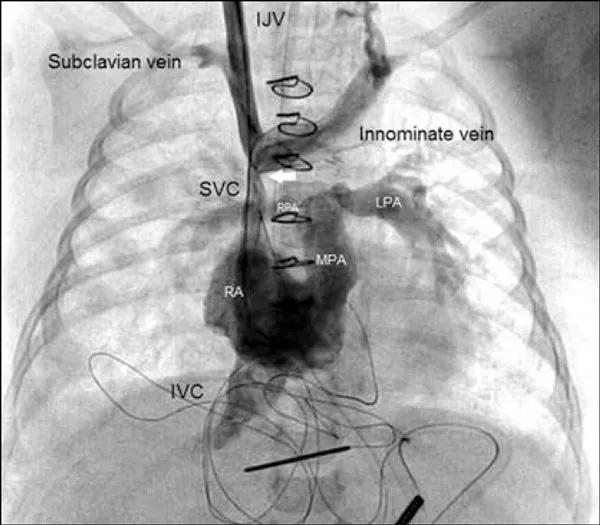
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/3aad7373-6374-488b-a8ac-50ccc7ff34df/18-HRT-117-Big-Rescues-CQD-Inset-4_jpg)
Figure 4. Venogram with injection of contrast bilaterally depicting the normal venous anatomy (not from the case patient).
A cardiothoracic surgeon was on hand for immediate cardiopulmonary bypass. The patient was heparinized, the ascending aorta was cannulated centrally, and the inferior vena cava (IVC) and right atrium were cannulated. Cooling was started for possible deep hypothermic circulatory arrest, and the heart was arrested with cardioplegia. All residual pacing leads were removed during cooling.
Access to the upper portion of the tear was very difficult and required periods of deep hypothermic circulatory arrest mixed with periods of low flow. The SVC was eventually reconstructed with autologous pericardium. Access to the upper parts was very difficult, and the native SVC was thin and friable. The innominate vein was ligated. A large amount of calcium was noted and removed from the right atrium.
Advertisement
The patient had an unremarkable postoperative course and was discharged home on postoperative day 11. Ejection fraction was still 30 percent, but she was asymptomatic and doing well at her most recent follow-up. She had no upper body edema, and a repeat postoperative CT demonstrated a patent but narrowed right SVC and good collaterals on the left side.
Although she never had an ICD shock during the 11 years of using the previous device, she still needs a defibrillator per guidelines but does not need pacing. She was subsequently implanted with a subcutaneous defibrillator to avoid endangering her repaired residual upper body venous system.
Over time, leads become more or less embedded in a sheath of scar tissue from chronic inflammation attached to the adjacent vascular wall and structures. This sheath eventually becomes calcified, which makes shearing off part of a vein a serious risk with lead removal. Such injuries are common even if not always recognized clinically, as our group recently reported.
The excessive calcium deposition in this patient, probably a result of her radiation therapy, contributed to the difficulty of the lead extraction.
Injuries around the SVC are notoriously challenging because of the complexity of circulation, as the confluence of veins results in force vectors in multiple directions. What may start as a small laceration can quickly get bigger and result in tamponade and circulatory collapse.
Acute hypotension during a procedure that doesn’t resolve within a few seconds is probably due to an injury. In our experience, the most common sites of injuries requiring emergency surgical or endovascular intervention are:
Advertisement
Performing lead extraction in a hybrid operating room can be critical. If we hadn’t had the capability to inflate the balloon and institute immediate cardiopulmonary bypass and surgery, the patient in this case would not have survived.
Cleveland Clinic instituted a protocol in 2014 for high-risk lead extractions that requires formal cardiac surgical backup. The protocol — which was roughly modeled on our protocol for cardiac surgery/interventional cardiology collaboration during transcatheter aortic valve replacement (TAVR) in response to CMS national coverage requirements for TAVR — is designed to minimize time to intervention in the event of a catastrophic complication from attempted transvenous extraction. High-risk cases are performed in a hybrid OR with surgical and cardiopulmonary bypass equipment on standby and a cardiac surgery team physically present until the “all clear” is given.
High-risk patients are evaluated by cardiac surgery before extraction to determine whether they are candidates for surgical backup in the hybrid OR. Candidates are generally selected because they are defined as high risk for perforation due to lead age (i.e., > 5 years) or because of previous cardiac surgery that would make surgical rescue more challenging.
For rescue candidates, a customized surgical plan is made, which may involve a full sternotomy or thoracotomy, depending on history, and appropriate pre-emptive measures are taken (e.g., wire access is placed for emergency peripheral cannulation). For those not deemed rescue candidates due to low probability of successful rescue (e.g., patients with very low ejection fraction, very advanced age or multiple prior cardiac surgical procedures), extraction proceeds without surgical backup if it is essential.
Advertisement
In our first three years of operating under this protocol, multiple patients have been rescued when central vascular injury resulting in tamponade has occurred. The protocol has also increased the level of interdisciplinary cooperation and involvement in lead extraction cases, which almost invariably leads to a higher level of patient care and safety.
Drs. Pettersson, Mick and Dash are with Cleveland Clinic’s Department of Thoracic and Cardiovascular Surgery. Dr. Wazni is Head of the Section of Electrophysiology and Pacing. Dr. Bauer is with the Department of Cardiothoracic Anesthesiology.
Advertisement
Modified-Bentall single-patch Konno enlargement (BeSPoKE) optimizes hemodynamics, facilitates future TAVR
Cleveland Clinic’s new dedicated program offers nuanced care for a newly recognized cardiovascular risk factor
Scenarios where experience-based management nuance can matter most
Introducing Krishna Aragam, MD, head of new integrated clinical and research programs in cardiovascular genomics
How Cleveland Clinic is using and testing TMVR systems and approaches
NIH-funded comparative trial will complete enrollment soon
How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR
Optimal management requires an experienced center