Patient with TP53 mutation achieves full response with targeted therapy
A previously health women in her 60s was diagnosed with high-risk mantle cell lymphoma. A year after enrolling in a clinical trial for a combination of monoclonal antibody, BTK inhibitor and BCL-2 inhibitor, she experienced complete remission with minimal side effects.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
A retired middle school teacher sought medical attention for enlarged lymph nodes in her neck. This led to a biopsy, which led to a diagnosis of mantle cell lymphoma. Her spleen was also quite enlarged at 27 centimeters, which is roughly double the normal size. In addition, she had pancytopenia from bone marrow involvement with mantle cell lymphoma.
Her community oncologist recommended initiating bendamustine and rituximab. However, after reading that some mantle cell lymphoma doesn’t respond to chemotherapy, she reached out to Cleveland Clinic Cancer Institute for a second opinion.
With a team of seven lymphoma specialists, Cleveland Clinic Cancer Institute has a depth of experience treating patients with this somewhat rare condition, which represents less than 10% of non-Hodgkin’s lymphoma.
Further evaluation and testing showed that the patient’s tumor harbored a TP53 mutation, which is typically associated with poor outcomes.
“In recent years, the hematology community has increasingly recognized that patients with mantle cell lymphoma who have TP53 mutations in their biopsy specimens at the time of diagnosis are at increased risk of failure with chemotherapy,” explains Director of the Lymphoid Malignancies Program and Staff Physician Brian T. Hill, MD, PhD. “Patients with these mutations tend to respond better to BTK inhibitors and BCL-2 inhibitors.”
The patient contacted the Institute’s Cancer Answer Line, whose goal is to arrange an appointment within seven days of the referral. “We really prioritize swift access to care because time is of the essence both in getting the disease under control and because patients are appropriately anxious,” says Dr. Hill. “Referring physicians also appreciate that if they need guidance with a challenging case, their patients can be seen quickly by one of our disease specialists.”
Advertisement
Based on the patient’s high-risk disease status and mutation status, Dr. Hill spoke with the patient and her husband about participating in a clinical trial where she would receive the monoclonal antibody rituximab in combination with the BTK inhibitor acalabrutinib and the BLC-2 inhibitor venetoclax. The goal was to provide her with treatment targeted for this mutation and spare her the side effects of traditional cytotoxic chemotherapy. The patient and her husband were eager to hear about alternatives to nonspecific chemotherapy, and she decided to participate in the trial.
“People often think of clinical trials as a last resort option and that’s not the case, especially in lymphoma,” says Dr. Hill. “We have so many good treatments and increasingly they’re being used in the frontline setting instead of chemotherapy. There are many clinical trial opportunities in the frontline setting as well as for patients who have relapsed. If your patient is at a fork in the road and you’re unsure of the right approach, I’d recommend speaking with a disease team specialist.”
After enrolling in the trial, the patient received IV rituximab monthly and the oral agents daily for roughly a year. All treatment was administered on an outpatient basis.
The patient tolerated the treatment remarkably well. Aside from minor bruising on her arms, which is common with BTK inhibitors, she had few side effects. There were mild changes to her platelet count but there were no neutropenia, infections or other cytopenias.
Advertisement
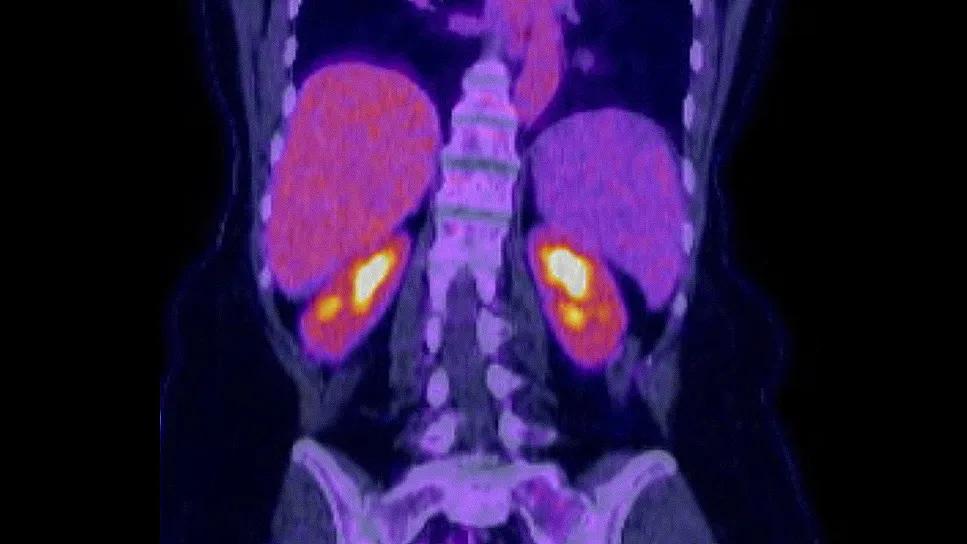
PET scans were performed at baseline and at the end of treatment. The scans showed that the patient entered a complete metabolic response. Minimal residual disease (MRD) testing showed no detected lymphoma. The plan is for her to continue acalabrutinib as a single agent maintenance therapy as part of the study.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/e59e790a-be1e-46b6-b50e-ee3cdf51a887/targeted-therapy-high-risk-mantle-cell-lymphoma-inset)
This “chemo free” approach is likely to be a standard of care for mantle cell lymphoma in the future, particularly for patients with TP53 mutation
Dr. Hill shared several takeaways from this case:
Consult with disease specialists for rare diseases. “Especially for less common diseases, it’s prudent to discuss cases like this with disease specialists,” says Dr. Hill. “The standard of care changes rapidly, and it’s difficult to stay up to date with all the changes in a community setting where you’re managing multiple diseases.”
Having a deep roster of specialists improves care. Dr. Hill specifically highlighted his outstanding colleagues at main campus, Drs. Caimi, Brooks, Winter, Jagadeesh, Dean and Bezerra, who all focus specifically on lymphoma. In addition to seeing patients and leading research studies, they run a weekly Lymphoma Tumor Board for complex cases. These insights are essential in a fast-moving field like lymphoma where the treatment landscape is shifting rapidly.
MRD testing is increasingly used clinically and in research studies to attain a more sensitive assessment of the depth of remission. MRD negativity correlates with a longer duration of response. “The patient achieved a deep remission, which we typically wouldn’t expect in this situation if she had received chemotherapy, explains Dr. Hill.”
Advertisement
The Institute’s hematopathologists keep up to date with the latest molecular testing and all indications. “TP53 testing for mantle cell lymphoma is now standard for Cleveland Clinic patients,” says Dr. Hill.
A strong research team makes a difference for patients. Dr. Winter, the principal investigator of the clinical trial, and the research nurses were instrumental in the patient’s care. Specifically, Sarah Billy, RN, made a big impression on the patient and her family for her attention to detail and close follow-up.
Advertisement
Advertisement

Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors

Early results show strong clinical benefit rates

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors