Can T-cell immunophenotyping help inform treatment decisions?
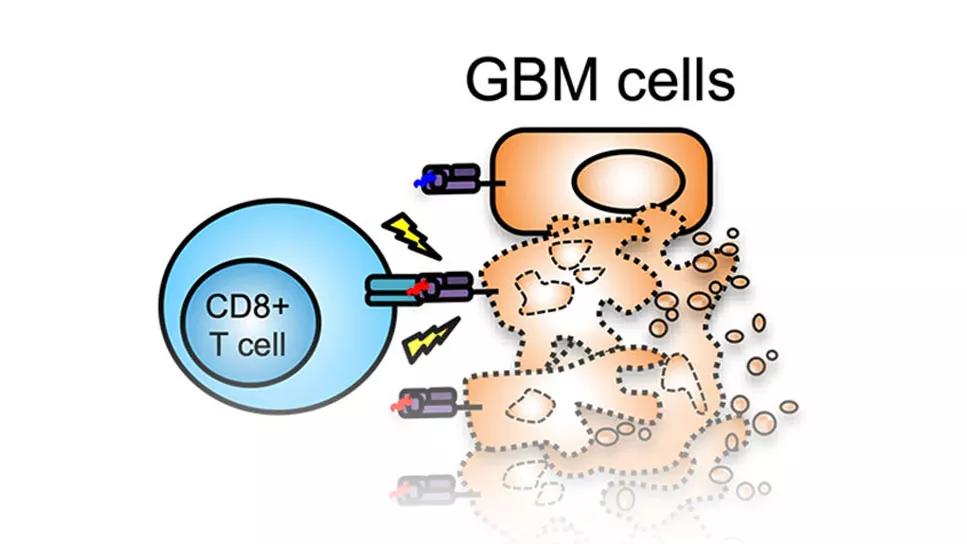
A study analyzing the phenotypic profile of T cells generated by adults with recurrent glioblastoma in response to combination therapy with SL-701, poly-ICLC and bevacizumab (SL-701+poly-ICLC+bevacizumab) found an association between survival and distinct subsets of T cells. The findings, presented at the Society for Neuro-Oncology’s 2022 annual meeting in November, shed new light on the potential clinical utility of analyzing the treatment-induced immune response.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“We noticed that patients who were living the longest were also the ones having the best immune response,” says lead investigator David Peereboom, MD, a medical oncologist with Cleveland Clinic’s Rose Ella Burkhardt Brain Tumor and Neuro-Oncology Center. “In this context, immune response refers to T-cell concentrations measured in the peripheral blood in direct response to the treatment. The purpose of this abstract was to try to delineate what was special about the particular T-cell populations associated with more durable responses.”
SL-701 is an immunotherapy made up of three peptides designed to create an immune response to three different antigens found on glioblastoma cells (Figure). “The three antigens are interleukin-13 receptor alpha-2, ephrin A2 and survivin,” Dr. Peereboom explains. All three antigens are found on glioma tumor cells at significantly higher levels than on normal cells.
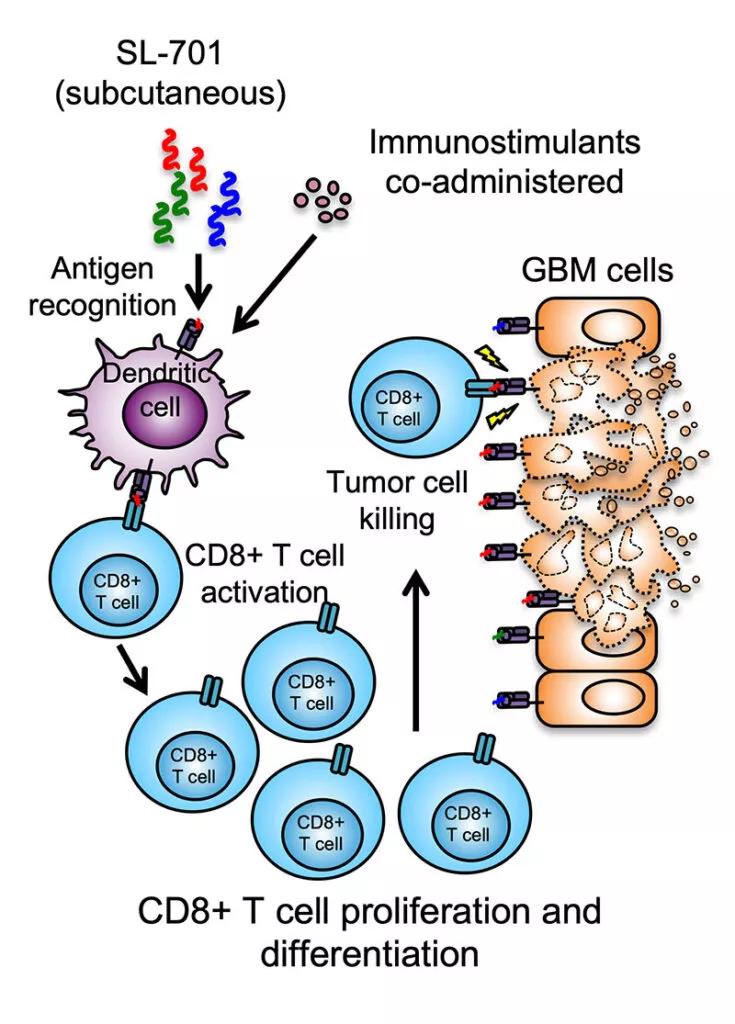
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/de6c26fe-e200-43de-bc67-eb5565909861/22-NEU-3409069-CQD-Inset-800x1115-1-735x1024_jpg)
Figure. Illustration of the mechanism of action of three-peptide systemic immunotherapy with SL-701. Used with permission of Stemline Therapeutics.
In 2015, SL-701 was granted orphan drug designation by the FDA for treatment of glioma based on positive findings from an earlier phase 1/2 trial of SL-701 in adults with high-grade gliomas. In the current phase 2 clinical trial of SL-701+poly-ICLC+bevacizumab in a cohort of adults with recurrent glioblastoma (NCT02078648), the 12-month overall survival (OS) was 50%.
Compared with an OS of approximately 20% to 38% achieved with bevacizumab, an OS of approximately 50% looks promising, Dr. Peereboom notes.
Advertisement
The current multicenter study used an 18-color flow cytometry analysis of peripheral blood samples from 27 patients included in stage 2 of the trial to analyze 18 different antibodies on T cells generated in response to treatment with SL-701. A machine learning immunophenotyping algorithm (TerraFlowä) was then used to identify T-cell phenotypes associated with clinical response. This approach allowed the investigators to delineate which combination of the 18 markers fared the best in terms of survival. Altogether, a total of 10,184 unique SL-701-induced phenotypes were analyzed.
From that total, the machine learning algorithm identified 16 core immunophenotypes that uniquely represent differences between patients with OS above or below 12 months. “Each phenotype represents a type of T cell with a different molecular signature,” Dr. Peereboom explains. Among the 16 core phenotypes, 10 were associated with OS greater than 12 months while six predicted OS of less than 12 months.
Of the 16 core phenotypes, 50% were CD8+ CD57+ CD107a+ PD-1– SL-701-specific T cells, also known as highly differentiated memory T cells primed for cytotoxicity. Immunophenotyping analysis further revealed the following:
Advertisement
Although the number of analyzed patient samples in the study was small, Dr. Peereboom is optimistic that the approach, if validated in a larger set of samples, may ultimately be used to predict a patient’s response to treatment.
“The idea would be to see if there is a way to pick out ahead of time those patients whose T cells would respond well,” he explains. “Then we might be able to tailor the treatment for a certain part of the glioblastoma patient population.”
Validating these early findings on SL-701-induced T-cell populations would be an important step before proceeding to a phase 3 clinical trial, Dr. Peereboom notes. “We also probably need to use more than just single-agent SL-701 for immune stimulation,” he adds. “We’ve been thinking of ways to boost the immune response, and that may involve adding in another drug, such as pembrolizumab, that would help the immune system.”
Advertisement
Advertisement

Phase 2 trials investigate sitagliptin and methimazole as adjuvant therapies

Advances in genomics, spinal fluid analysis, wearable-based patient monitoring and more

Researchers use AI tools to compare clinical events with continuous patient monitoring

Combining dual inhibition with anti-PD1 therapy yielded >60% rate of complete tumor regression

Cleveland Clinic researchers pursue answers on basic science and clinical fronts
New research from Cleveland Clinic helps explain why these tumors are so refractory to treatment, and suggests new therapeutic avenues

Presurgical planning and careful consideration of pathology are key to achieving benefits
Study demonstrates its role in tumor lethality, raises prospect of therapeutic targets