Findings promise potential wireless option for majority of patients who need pacing
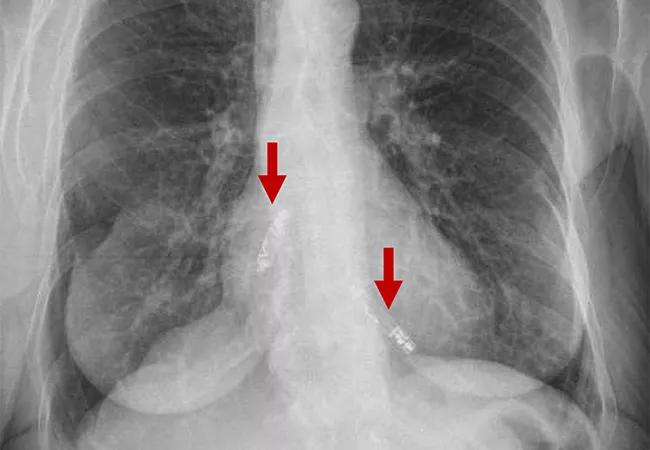
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/db07f9bb-6aa1-4acd-ab8c-d3ee4e186814/23-HVI-3941962-CQD-650x450-1_jpg)
imaging study showing two leadless pacemakers in upper and lower chambers of heart
A first-of-kind dual-chamber leadless pacemaker system demonstrated promising results in the first three months following implantation, reported investigators with the ongoing international Aveir DR i2i Study. The findings were recently presented in a late-breaking clinical trials session at Heart Rhythm 2023 and simultaneously published online in the New England Journal of Medicine.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
In the trial, two miniaturized leadless pacemakers were independently implanted into the heart’s upper and lower chambers via a catheter through the femoral vein and were able to provide beat-to-beat wireless communication to synchronize the heart’s pumping action.
Results of the study showed that implantation was successful in 98.3% of patients, and beat-to-beat wireless bidirectional communication with upper and lower chamber synchrony was achieved in 97.3% of patients. Study participants had standard indications for dual-chamber pacing.
“These outcomes exceeded our performance goals, meaning more patients who require pacing support may soon have a less-invasive option, and we can now offer a more targeted approach,” says co-principal investigator Daniel Cantillon, MD, who presented the study results. “Until now, leadless pacing has been limited to stimulating the heart’s lower chambers. This new system can pace both upper and lower chambers using beat-to-beat wireless communication, which expands the benefits of leadless pacing to serve the needs of a majority of patients worldwide.”
In March 2022, Dr. Cantillon performed the study’s first implantation of the system in a U.S. patient at Cleveland Clinic, where he served until recently as Research Director in the Section of Cardiac Electrophysiology and Pacing. He now works for Masimo, a U.S. medical technology company. Cleveland Clinic continues as a participating center in the study.
“We are proud to have contributed to this latest advancement in dual-chamber leadless pacemaker technology,” says Oussama Wazni, MD, MBA, Section Head of Cardiac Electrophysiology and Pacing at Cleveland Clinic. “Since dual-chamber pacemakers are the predominant type of CIEDs implanted in the U.S., the potential impact of these findings for reducing patient morbidity is significant.”
Advertisement
The investigational dual-chamber leadless pacemaker system, known as Aveir™ DR, is an evolution from earlier single-chamber leadless pacemakers, which were first introduced in 2014. Both single- and dual-chamber leadless devices are designed to avoid the complications of traditional transvenous pacemakers ― i.e., bleeding or infection in the implantation pocket and dislodgement, fracture or blood clotting around the lead. “Such complications affect one in six patients within three years of implantation, often causing substantial morbidity,” Dr. Cantillon notes.
Leadless single-chamber pacemakers for the right ventricle have been shown to reduce complications relative to transvenous pacemakers, but they are unable to offer atrial pacing or consistent AV synchrony. This limits their potential use to just 10% to 20% of patients with a pacemaker indication.
In contrast, the most common pacemaker indications — sinus node dysfunction and heart block — can be managed by dual-chamber devices, prompting development of the leadless dual-chamber design of the Aveir DR to meet more patient needs without the risks of transvenous leads.
The Aveir DR i2i Study is a prospective, single-group investigation of patients with a standard indication for dual-chamber pacing. Planned enrollment is up to 550 patients at up to 85 sites worldwide; the results reported so far are for the first 300 enrollees, all of whom underwent an implantation attempt from February through August 2022. The vast majority had either sinus node dysfunction (n = 190; 63.3%) or AV block (n = 100; 33.3%) as their primary pacing indication.
Advertisement
All implantations were done as a single procedure. Fluoroscopy was required in all cases, with intracardiac echocardiography as an option to assist in guiding implantation.
Successful implantation was achieved (i.e., with implant-to-implant communication between two functioning pacemakers) in 295 patients (98.3%).
The primary safety endpoint — freedom from complications (device- or procedure-related serious adverse events) at 90 days — was achieved in 271 patients (90.3%), surpassing the performance goal of 78% (P < 0.001). The other 29 patients experienced a total of 35 complications, 28 of which occurred within two days after implantation.
The most common complications were procedural atrial fibrillation (n = 9) and intraprocedural (n = 5) or postprocedural (n = 5) dislodgement of a leadless pacemaker. The pacemakers dislodged intraprocedurally were all retrieved and successfully reimplanted during the initial procedure. Those dislodged postprocedurally were retrieved percutaneously.
No procedure- or device-related deaths occurred. The researchers noted that the incidence of acute complications — including pericardial effusion in two patients (0.7%) — was comparable to that in studies of transvenous dual-chamber pacemakers.
The first primary performance endpoint — a combination of adequate atrial capture threshold and sensing amplitude at three months — was achieved in 90.2% of patients, exceeding the performance goal of 82.5% (P < 0.001).
The second primary performance endpoint — ≥70% AV synchrony while seated, measured at three months — was met in 97.3% of patients, surpassing the performance goal of 83% (P < 0.001).
Advertisement
Dr. Cantillon says these initial results are encouraging but that long-term follow-up is needed. Assessment in the study will continue at six and 12 months after implantation and every six months thereafter. The study is also limited by its single-arm, noncontrolled design.
If the current findings are maintained through rigorous long-term follow-up, the researcher note, potential use cases for the technology include atrial-only and ventricular-only pacing indications as well as service as a combined AV-synchronous system.
“Eventual approval of a dual-chamber leadless pacemaker would provide a wireless pacing option to the vast majority of patients who require pacing,” Dr. Cantillon concludes. “That promises to reduce the long-term risks of infection and lead malfunction with conventional transvenous devices.”
Dr. Cantillon reports that he has financial interests with Abbott Medical related to speaking, consulting and service on scientific advisory boards (Abbott is developing the Aveir DR). Dr. Cantillon is now an employee of Masimo, a U.S. medical technology company.
Advertisement
Advertisement
Medical and surgical perspectives on current and emerging uses of ECMO and Impella
Least-invasive open-heart AVR option to date yielded rapid recovery in all cases
Launch of the tool promises to reshape quality assessment across the specialty
Lead dwell time and manufacturer emerge as independent predictors of success in registry study
The case for a thoughtful approach to CTO and minimally invasive options for CABG
How the tool could help physicians alter management in real time
Preoperative Impella 5.5 placement can provide a critical safety net for high-risk patients
Technique may lay groundwork for personalized decision-making in procedural intervention