Further study needed to assess potential role in select subgroups
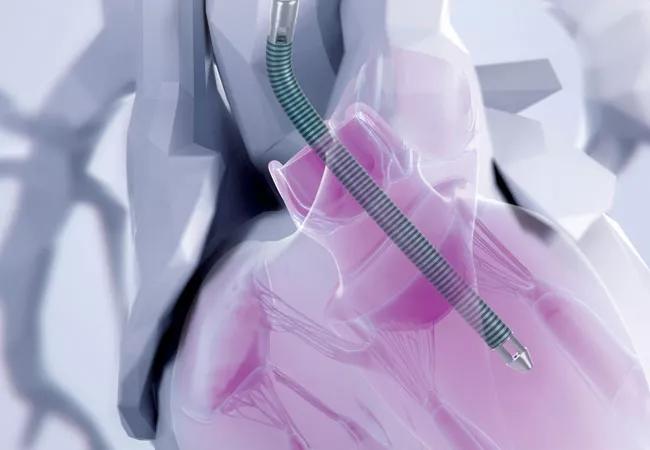
Use of the Impella 5.0® or 5.5® device to provide full left ventricular hemodynamic support for high-risk advanced heart failure patients undergoing ablation for recurrent ventricular tachycardia (VT) did not improve outcomes and was associated with a high rate of surgical and procedure-related complications. So reported Cleveland Clinic investigators in a late-breaking clinical trials session at Heart Rhythm 2023.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The retrospective propensity-matched analysis, which was simultaneously published in the Journal of the American College of Cardiology, represents the first-in-human experience of this procedural approach in this setting.
The temporary left ventricular assist device was surgically implanted in 41 consecutive patients at high risk of periprocedural acute hemodynamic compensation; 16 received Impella 5.0 and 25 received Impella 5.5. Their outcomes were compared with those of a historical propensity-matched control group of 41 patients with similar baseline risks who underwent ablation without mechanical hemodynamic support.
During median follow-up of 423 days, 22 Impella recipients (54%) and 24 control patients (59%) reached the primary endpoint, a composite of all-cause death, permanent left ventricular assist device (LVAD) implant or heart transplant. The rate of recurrent VT/ventricular fibrillation, the secondary endpoint, also was similar between the two groups.
“We thought that supporting patients with a high-flow device would improve outcomes in terms of 30-day and one-year mortality, since prior reports suggested it would,” says senior investigator Pasquale Santangeli, MD, PhD, Director of the VT Program in Cleveland Clinic’s Section of Cardiac Electrophysiology and Pacing. “Yet we found it had no significant impact. Numerically, there was a significant reduction in periprocedural acute hemodynamic decompensation events in the device arm, but this benefit was offset by a tenfold higher rate of complications with the Impella device.”
Advertisement
Why the procedure did not meet expectations is unclear. However, Dr. Santangeli describes the study cohort “the sickest group of patients we could have possibly enrolled.” He and first author Jakub Sroubek, MD, PhD, agree that the patients’ poor condition likely explains why Impella had little impact on long-term outcomes.
“Many of these patients have comorbidities that, by definition, make them high risk, and they end up succumbing to advanced heart failure or another illness,” says Dr. Sroubek, a staff physician in the Section of Cardiac Electrophysiology and Pacing.
The impact of Impella was not entirely unfavorable, however, as there was a trend toward a reduction of periprocedural acute hemodynamic decompensation events, which allowed more detailed VT mapping.
“Impella enabled us to ablate the VT better and map the patient more easily,” Dr. Sroubek notes. “If you look solely at the outcomes when patients left the electrophysiology lab, they were slightly better with Impella. Unfortunately, that did not necessarily mean the patients ultimately did well. Preventing acute hemodynamic decompensation should have improved short-term outcomes, but 30-day mortality was the same with and without Impella.”
Seven of the 22 Impella recipients reaching the primary endpoint experienced bleeding complications, and these were largely related to surgical implantation of the device near the shoulder.
“These were not necessarily life-threatening complications, but the Impella is a large device inserted by surgeons, and that alone is another procedure with risks,” Dr. Sroubek observes. “Often the bleeding was treatable and curable, but it prolonged patients’ hospitalization.”
Advertisement
Absolutely not, say Drs. Santangeli and Sroubek. Both feel there are patient subgroups who could benefit.
“We will continue to use Impella in patients with advanced heart failure and nearly incessant VT that cannot be controlled with medications,” says Dr. Santangeli. “These individuals have a high rate of acute hemodynamic compensation during ablation, and I am confident we will find benefit in this population.”
Impella also may show value for reducing ablation failure in some less-sick patients with VT.
“When we don’t know where the VT is coming from and there is no way to identify where the VT substrate is located, it would be helpful to have the patient in continuous VT in order to ablate it,” Dr. Sroubek says. “However, some patients are not hemodynamically stable in VT and require external shock to terminate the arrhythmia shortly after it is induced. This does not allow enough time for VT circuit mapping. In such circumstances, an Impella device could provide enough support to sustain the VT longitudinally and allow for full mapping. These scenarios may constitute a very reasonable use for Impella. In fact, I encounter select cases like this relatively frequently.”
As Cleveland Clinic continues investigating the potential benefit of providing acute hemodynamic support during VT ablation, Dr. Santangeli would like to see changes made in the Impella device itself.
“Ideally, the device should be made smaller in order to reduce the risk of complications,” he says. “Using it in the right patients — those at high risk of decompensation and with high burden of VT — should help get them through the procedure.”
Advertisement
While the Cleveland Clinic team remains interested in exploring the value of Impella for VT ablation support in certain subgroups, they say their study’s overall results clearly call for some caution.
“Using the device in this setting comes at a cost, and that is complications,” Dr. Sroubek says. “It doesn’t seem to prolong life or freedom from VT in all comers, so using it broadly to treat high-risk patients does not pan out.”
“Without long-term benefit, and with a high possibility of complications, the juice may not be worth the squeeze,” Dr. Santangeli adds.
“The Impella 5.5 device has revolutionized the care of patients in decompensated heart failure as a form of temporary mechanical circulatory support (MCS),” says study co-author Edward Soltesz, MD, MPH, Surgical Director of Cleveland Clinic’s Kaufman Center for Heart Failure Treatment and Recovery. “It provides a significant iterative improvement over the previous Impella 5.0 transvalvular axial flow device. Our program and others have used it for temporary support to transition high-risk patients with low ejection fraction through complex heart surgery with excellent success and very low periprocedural morbidity. The Impella 5.5 has also improved outcomes in patients with cardiogenic shock relative to other forms of temporary MCS that do not directly unload the left ventricle.”
He adds that while the Impella family of devices offers significant advantages over other forms of support, such as intra-aortic balloon pump and extracorporeal membrane oxygenation, patient selection is crucial for good outcomes. “The patients in this study represented some of the sickest patients our teams care for,” Dr. Soltesz notes. “The ultimate question in this population is not whether the Impella actually is better than no form of temporary MCS, but rather whether such patients should not be treated with high-risk ablation at all but instead directly undergo advanced therapies such as LVAD implant or transplant. Device-related complications were minimal except for local bleeding in the axillary site related to muscle bleeding, not access-site bleeding. The lack of benefit in device-supported patients derives from progression of their advanced heart failure. The data from this study should provide one clear message: all patients with lower ejection fraction undergoing complex VT ablation should have a full advanced therapies evaluation to determine whether ablation is actually the best course of action.”
Advertisement
Advertisement
Procedure allows for safer epicardial VT mapping and ablation
Study finds that in the absence of ACS, the yield is low and outcomes are unaffected
New technology aids a Cleveland Clinic London patient with a complex arrhythmia

Multidisciplinary care can make arthroplasty a safe option even for patients with low ejection fraction

Experts are challenging the one-size-fits-all paradigm

High-risk procedure prepares patient for lifesaving heart surgery

Nurses play a vital role in helping patients manage the chronic disease in inpatient and outpatient settings

Check out our latest data in these core cardiovascular areas