Focus is on giving patients a better imaging experience while acquiring more data
Current-generation cardiac magnetic resonance imaging (CMR) exams involve a lengthy and cumbersome process. Patients must repeatedly hold their breath throughout the hourlong scan, which can be exhausting or even prohibitive for many in poor health. In addition, the technologist must continuously adjust controls on a panel similar to those in a jet’s cockpit. And although CMR provides more information than other cardiac imaging modalities, a considerable amount of acquired data is unexploited because of processing limitations.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“CMR is the most valuable imaging technique for diagnosing and monitoring cardiac disease, but because of its complexity and poor patient experience, it is underused,” says Deborah Kwon, MD, Director of Cardiac MRI at Cleveland Clinic, who is spearheading new cardiovascular imaging initiatives under the Advanced Imaging and Simulation Center. “We are driving efforts to create high-performance CMR by improving efficiency and patient comfort while simultaneously augmenting image quality and accuracy of diagnosis.”
One central initiative is the Advanced Imaging and Simulation Center for MRI, led by Christopher Nguyen, PhD, Director of MRI Research, who arrived at Cleveland Clinic in April 2022 from Harvard Medical School. An expert in developing novel CMR techniques, Dr. Nguyen and his team are focused on several major innovations, including those discussed below. Central to their efforts is a new 3T MRI scanner at Cleveland Clinic dedicated exclusively to cardiovascular research under Dr. Nguyen’s leadership.
Conducting a CMR exam requires extensive training, as technologists must make hundreds of decisions during a single session. The complexity introduces much variability among different technologists — and even among exams conducted by the same technologist — which can render comparisons inexact for clinical and research purposes.
“We are taking advantage of new technical advances in hardware and software to simplify the CMR interface,” says Dr. Nguyen. “We aim to develop a single push-button exam, using data to allow the machine to run on ‘autopilot’ for tasks currently performed manually.”
Advertisement
From the patient’s perspective, CMR exams today are “like running a marathon,” Dr. Nguyen observes. Because capturing images is slow and the heart beats fast, breath-holding is required while each of about 100 images is taken. The next-generation scanner will use a continuous acquisition approach, capturing rather than avoiding motion (see Nat Biomed Eng. 2018;2(4):215-226 and Magn Reson Med. 2022;87(1):474-487) and yielding more revealing images (Figure 1). By 2023, Dr. Nguyen expects to bring the exam time down to 20 to 30 minutes with the patient breathing freely throughout. He reports that when he’s testing the prototype, he instructs patients to “just take a nap!”
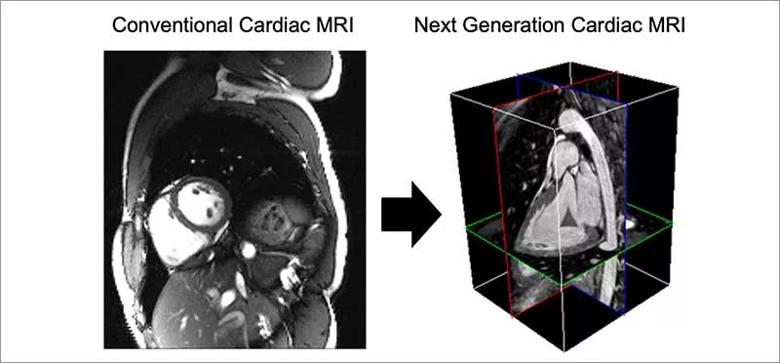
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2d927ace-4acc-4d9a-be82-8be276134bdd/Inset-1-2_jpg)
Figure 1. Evolution of the cardiac MRI (CMR) exam. Conventional CMR exams acquire thick 2D slices from multiple views and require the patient to hold their breath numerous times. The next-generation CMR exam will be shorter while collecting a complete dynamic 3D volume at each phase of the cardiac cycle under completely free breathing conditions.
With the help of deep learning and noise-reduction techniques, the new dedicated MRI machine will be able to incorporate the massive amount of data it acquires, providing images with much more information than offered by current exams (see Front Physiol. 2021;12:694940). Dr. Nguyen explains that while a typical MRI obtains 1 to 2 gigabytes of data during a scan, the new scanner can acquire 100 gigabytes.
This wealth of data will not only provide additional clinical information to help the patient, but it can also be used to help others — an unusual circumstance in clinical imaging. Data related to multiple anatomic and functional parameters from hundreds of similar hearts can be combined to create a “digital twin.” Such models can be used in multiple ways to inform research and personalized clinical care (see Figure 2). For instance, various treatments can be compared using outcomes of a conglomerate of similar hearts from patients who received each therapy. This can greatly benefit research and help determine the best path forward for an individual patient.
Advertisement
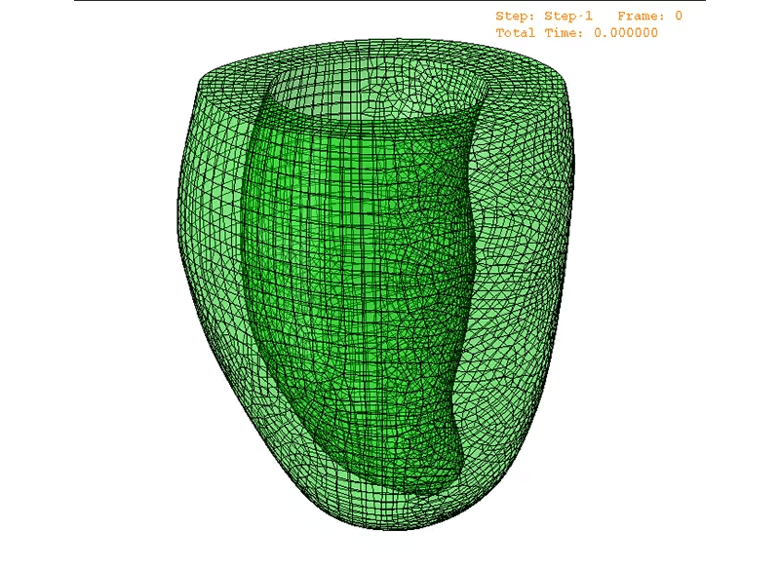
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/1133d325-bd5d-4eec-9270-d72bc38e49d8/Inset-2-animated-1_gif)
Figure 2. A representative patient-specific CMR-based biomechanical mesh from digital twin technology. This mesh can simulate the patient’s heart to recapitulate their cardiac motion, hemodynamics and remodeling.
Digital twin technology can also give patients a glimpse into their future. “We can show patients a ‘Tale of Two Hearts,’” Dr. Nguyen explains. “We can say, ‘This is what your heart will probably look like in 10 years if you continue smoking, and this is how it will likely look if you quit.’”
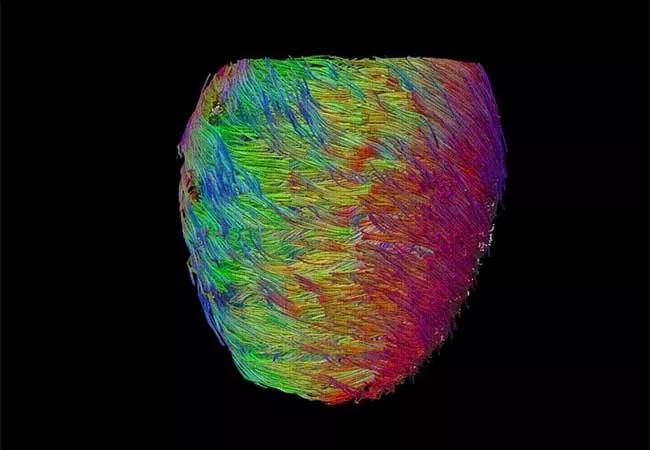
The imaging acquisition and processing advances are also enabling new developments on a micropathological level by using a novel diffusion tensor CMR technique pioneered by Dr. Nguyen (Figure 3). Like finding cracks in a building, this new technique can detect aberrant helical cardiac muscle fiber architecture caused by early disease before other imaging modalities can detect it (see JACC Basic Transl Sci. 2018;3(1):97-109 and Radiol Cardiothorac Imaging. 2021;3(3):e200580). Visualizing microstructure can also be a boon for electrophysiologists: because atrial fiber orientation reflects electroconductivity direction, the ability to visualize and map electrical pathways can help identify ablation targets to treat atrial fibrillation.
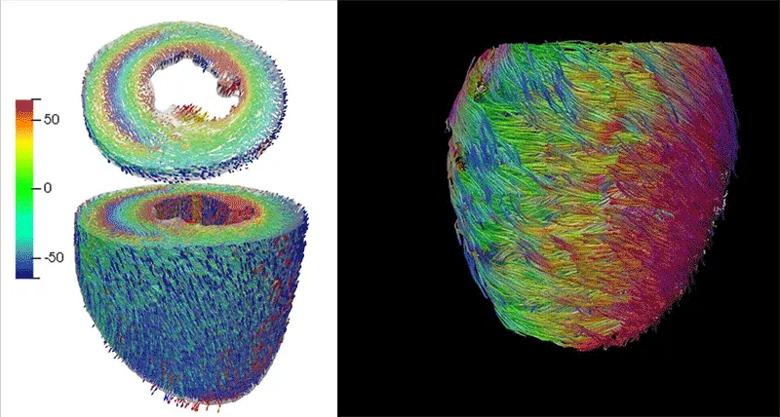
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2e64b5ef-59e8-4293-9ae3-bf42ea39dd81/Inset3-animation2_gif)
Figure 3. Microstructural imaging enabled by diffusion tensor CMR. By tracking diffusion of water molecules, microstructure on the level of 100 µm can be characterized, revealing the underlying myocardial helical fiber architecture or its helicity. The helicity maps (left) show a smooth transition from endocardial right-handed helical fiber structure (red) to epicardial left-handed helical fiber structure (blue).
Advertisement
Dr. Kwon notes that this innovative microstructural imaging and machine learning will also be useful for advancing research and has the potential to play an important role in settling controversies. She cites the differing results of the COAPT and MITRA-FR trials of transcatheter mitral valve repair with the MitraClip device, with the former finding improved outcomes using the device relative to medical therapy alone and the latter finding no benefit.
“Perhaps certain patterns of remodeling that result from the underlying cardiomyopathy — too subtle to detect by current evaluation methods — determine whether only some patients benefit from this device,” says Dr. Kwon. “Current guidelines call for assessment by echocardiography before MitraClip is employed, but micropathology may provide the ability to derive more optimal selection criteria to better predict outcomes.”
Dr. Kwon describes the Advanced Imaging and Simulation Center for MRI as a “team of teams,” with close collaboration among engineers, technologists, data scientists, clinical informatics specialists and physicians in various disciplines. This allows for seamless interchange between the research and clinical realms and the promotion of clinical relevance and rapid translation.
She adds that the excitement surrounding the new imaging capabilities extends to their potential to spur further innovation.
“The new CMR capabilities will also help drive device development and improved techniques,” she says. “By more efficiently providing better images, combined with digital twin technology and machine learning, the new innovations will launch the next generation of advances in cardiac care.”
Advertisement
To learn more about this work, readers can contact Dr. Nguyen at nguyenc6@ccf.org and Dr. Kwon at kwond@ccf.org.
Advertisement

Scenarios where experience-based management nuance can matter most

Introducing Krishna Aragam, MD, head of new integrated clinical and research programs in cardiovascular genomics

How Cleveland Clinic is using and testing TMVR systems and approaches

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients