Multidisciplinary assessment helps minimize poor outcomes
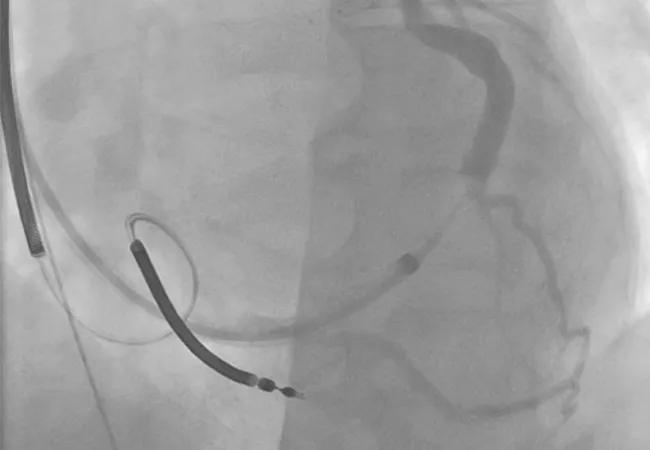
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/a1ea5d4d-cfb7-4f9d-b869-b62428bcf321/16-HRT-2453-CRT-CQD-650p_jpg)
Nonresponse to CRT: A Case Study in Systematic Troubleshooting
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Mr. JL is a 48-year-old African-American male who until one year ago had a medical history significant only for obstructive sleep apnea, for which he intermittently used continuous positive airway pressure therapy. He noted at his job as a construction worker that he was becoming progressively short of breath during routine tasks and was eventually referred to a cardiologist in his local community. Echocardiography showed significant left ventricular dysfunction, with a left ventricular ejection fraction (LVEF) of 20 percent. Left heart catheterization showed normal coronary arteries. He was started on carvedilol 12.5 mg twice daily, lisinopril 10 mg daily and furosemide 80 mg daily. He continued to have significant symptoms but was able to return to work.
Repeat echocardiography six months after diagnosis revealed his LVEF to still be 20 percent despite optimal medical therapy. As a result, he was referred for implantation of a biventricular defibrillator. A percutaneous left ventricular lead could not be placed, so he was referred for surgical left ventricular lead placement, which he underwent successfully at an outside institution. He was discharged in stable condition.
Over the next six months, his symptoms continued to worsen. He could no longer work and was hospitalized for heart failure. When a follow-up echocardiogram showed that his LVEF had dropped to 15 percent, he was referred to Cleveland Clinic’s Cardiac Resynchronization Therapy Optimization Clinic.
This specialized clinic (detailed in this earlier post) employs an algorithmic approach for patients who have not responded to cardiac resynchronization therapy (CRT) as much as was expected in terms of echocardiographic and/or symptomatic improvement. The clinic uses a multidisciplinary model — with input from electrophysiologists, heart failure and imaging cardiologists, cardiothoracic surgeons and sometimes other subspecialists — to discuss findings from the algorithm-driven examination, determine potential reasons for inadequate CRT response and establish a treatment plan to optimize response and long-term outcomes.
Advertisement
Physical examination revealed volume overload, a nonischemic cardiomyopathy of only one year’s duration, grade 2+ lower extremity edema and faint bibasilar rales. Comprehensive device evaluation showed 99 percent biventricular pacing with excellent function of all leads. Twelve-lead ECG was performed (Figure 1, left panel) and showed a paced pattern with a deep S wave in lead V1, raising potential questions about lead position. Adequate capture of the left ventricular lead was confirmed, and no anodal stimulation was noted.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/41e95036-6097-4b4a-933c-92d4f15a0a85/16-HRT-2453-CRT-CQD-Inset-1_jpg)
Figure 1. The patient’s ECGs upon presentation (top) and with suppression of biventricular pacing (bottom).
Suppression of pacing revealed a wide underlying left bundle branch block (Figure 1, right panel), suggesting a highly conducive substrate for response to CRT. When chest X-ray showed an anterior/anterolateral surgically placed left ventricular lead (Figure 2), this was determined to be the likely culprit for CRT nonresponse following discussion at a multidisciplinary conference.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/f81352ff-1575-49c1-afa4-77d951984c69/16-HRT-2453-CRT-CQD-Inset-2_jpg)
Figure 2. Lateral chest X-ray showing an anterior/anterolateral left ventricular pacing lead.
A copy of the patient’s coronary sinus venogram was then obtained (Figure 3) to determine whether another attempt at percutaneous placement would be reasonable. The venogram showed two potential targets:
Advertisement

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/ef04d53b-868c-4aa1-8332-fc1459d11f35/16-HRT-2453-CRT-CQD-Inset-3_jpg)
Figure 3. Coronary sinus venogram showing potential targets for percutaneous coronary sinus lead placement at the lateral and posterolateral branches.
A repeat attempt at surgical lead placement was considered, but consultation with cardiothoracic surgery colleagues determined that the patient was not an ideal candidate due to anatomical constraints. A CRT optimization echocardiogram with three-dimensional strain imaging was performed, showing worsened cardiac performance with CRT on versus off.
In view of these collective findings, the CRT Optimization Clinic’s multidisciplinary team made a final recommendation to proceed with a repeat attempt at percutaneous coronary sinus lead placement targeting the posterolateral branch. The recommendation calls for interventional cardiology assistance to perform venoplasty of the stenotic midsection of the vessel to allow passage of the lead. As of this writing, the procedure has been scheduled but not yet performed.
CRT has been a godsend for individuals with advanced heart failure, but as many as one-third of patients fail to respond to CRT. In the absence of a thorough multidisciplinary assessment, these patients are at increased risk for poor exercise tolerance, repeat hospitalizations and early death.
At Cleveland Clinic, we recommend that all patients who receive a CRT device be re-evaluated with an echocardiogram within six to nine months. At that point, patients who have realized little to no benefit should undergo “troubleshooting” of their care to systematically identify possible reasons for nonresponse. We have created our CRT Optimization Clinic, one of the few programs of its type in the nation, to deliver that troubleshooting in a way that ensures a collaborative, multidisciplinary approach guided by a thorough, algorithm-directed evaluation.
Advertisement
The clinic’s collaborative ethos extends to fully include patients and their referring cardiologists. If a major intervention is required (e.g., reoperation to move a lead), the recommendation is discussed with the referring clinician and a plan is formulated. If only minor device changes are needed or no significant interventions are possible, patients are followed by their referring cardiologist with Cleveland Clinic’s CRT Optimization Clinic acting in a consultative role.
Dr. Rickard is an electrophysiologist and Director of Cleveland Clinic’s CRT Program.
Advertisement
Advertisement
Modified-Bentall single-patch Konno enlargement (BeSPoKE) optimizes hemodynamics, facilitates future TAVR
Cleveland Clinic’s new dedicated program offers nuanced care for a newly recognized cardiovascular risk factor
Scenarios where experience-based management nuance can matter most
Introducing Krishna Aragam, MD, head of new integrated clinical and research programs in cardiovascular genomics
How Cleveland Clinic is using and testing TMVR systems and approaches
NIH-funded comparative trial will complete enrollment soon
How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR
Optimal management requires an experienced center