Current screening strategies may be missing many patients who have the disease
By Ali Mehdi, MD, MEd; Pratibha Rao, MD, MPH; and George Thomas, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
This article is reprinted from the April 2021 issue of Cleveland Clinic Journal of Medicine (2021;88[4]:221-227).
Primary aldosteronism is the most common endocrine cause of hypertension and is often associated with treatment-resistant hypertension.1 It is characterized by autonomous aldosterone production independent of renin activity, potassium level or volume status. Its prevalence has been debated, and studies are fraught with limitations, including reliance on tests that do not confirm the diagnosis. It has been identified as a public health issue,2 and the urgency in identifying and treating it is heightened by its strong association with adverse kidney and cardiovascular outcomes.1,3,4
Multiple studies have pointed to limitations of our current screening strategies, which may be missing a great many patients who have the disease.5,6 In particular, newer evidence highlights shortcomings of the long-accepted screening strategy that uses the spot aldosterone-to-renin ratio.7
In this commentary, we review the current understanding of primary aldosteronism and the current landscape of screening practices. We will highlight the emerging evidence suggesting that renin-independent hyperaldosteronism is best viewed as a continuum that extends across the spectrum of blood pressure severity. We also describe a proposed approach to optimize case detection of primary aldosteronism.
Primary aldosteronism was first described in 1954 by Dr. Jerome Conn,8 who noted a syndrome of periodic muscle weakness, hypokalemia, alkalosis and hypertension with elevated urinary aldosterone levels.
Advertisement
Subsequently, hypokalemia came to be viewed as an important component of this condition, to the point that it became almost a sine qua non for the diagnosis. Although primary aldosteronism was classically suspected in patients presenting with the triad of hypertension, hypokalemia and metabolic alkalosis, this phenotype likely describes an extreme form of the condition. In fact, only 9% to 37% of patients with primary aldosteronism have hypokalemia, and the most common presentation is normokalemic hypertension.2 Hypokalemia often alerts the clinician to a possible diagnosis of primary aldosteronism. However, the absence of hypokalemia holds a poor negative predictive value for diagnosis and should not be used to exclude the presence of the disorder.
The prevalence of primary aldosteronism varies significantly among published reports, and its true prevalence remains unclear. What is becoming clear, however, is that the old notion that this condition accounts for fewer than 1% of cases of mild to moderate hypertension is not true.2 The variability in the reported prevalence stems primarily from the populations being studied (i.e., the general population vs. hypertensive patients vs. those with resistant hypertension). Most experts now accept that the prevalence of primary aldosteronism is between 5% and 10% among all hypertensive patients, and up to 20% in those with resistant hypertension.9,10
A major challenge in studying the epidemiology of primary aldosteronism is that there is no gold standard for diagnosing it. The default standard has been the aldosterone-renin ratio, but the diagnostic cutoff varies across practices and has not been validated in prospective trials. Liberalizing or restricting the diagnostic cutoffs can have significant effects on the perceived prevalence.
Advertisement
Step 1: Screening with the ratio of aldosterone to renin. The current Endocrine Society guidelines for detecting and diagnosing primary aldosteronism, published in 2016, recommend the aldosterone-renin ratio as the most reliable screening test.2 The reporting should include the plasma aldosterone concentration and the plasma renin activity or direct renin concentration, along with the aldosterone-renin ratio. Patients are at high risk of primary aldosteronism and should be screened for it if they have any of the following:
The guidelines acknowledge that the threshold defining a high aldosterone-renin ratio (and thus a positive screen) varies by practice, but the most common one is a ratio of 30 ng/dL per ng/mL/hour or higher with a plasma aldosterone concentration of 15 ng/dL or higher. (See Table 1, available here in the Cleveland Clinic Journal of Medicine version of this article.)2,11
In the interest of convenience and automation, many laboratories have switched from the traditional plasma renin activity assay to a direct renin concentration assay. While the two assays generally correlate well with one another, conversion factors vary slightly among different laboratories. A discussion of the advantages and disadvantages of each assay is beyond the scope of this commentary. Practitioners, however, must consult with their laboratory when interpreting direct renin concentration and converting to plasma renin activity.
Advertisement
Step 2: Confirmatory testing. Patients who have a positive (high) result on the aldosterone-renin ratio screening test should then undergo a confirmatory diagnostic test to definitively confirm or exclude primary aldosteronism.
The guidelines describe four confirmatory testing procedures: oral sodium suppression, saline infusion suppression, fludrocortisone suppression and captopril challenge test. All these tests hinge on the premise that aldosterone should normally be suppressed with the above interventions. Persistent aldosterone production would be indicative of autonomous renin-independent aldosteronism, thus confirming primary aldosteronism.
The cutoffs used to define a positive result on a confirmatory test again vary by practice. (See Table 1, available here in the Cleveland Clinic Journal of Medicine version of this article.)2,11
The guidelines note an exception to the need for the confirmatory step. Patients with spontaneous hypokalemia, suppressed renin and a plasma aldosterone concentration higher than 20 ng/mL on the screening test can be confidently given the diagnosis of primary aldosteronism without confirmatory testing.
After the diagnosis of primary aldosteronism is confirmed, patients proceed with subtype evaluation, including computed tomography of the adrenal glands and, potentially, adrenal venous sampling to further classify the primary aldosteronism as due to adenoma versus hyperplasia. This information can then guide the therapeutic strategy of surgery (adrenalectomy) versus medical therapy with a mineralocorticoid receptor antagonist. Our commentary is limited to case detection, and we acknowledge that subtype classification and treatment of primary aldosteronism is another area of intense discussion and yet another element in the changing landscape of primary aldosteronism.
Advertisement
Current guidelines recommend screening for primary aldosteronism only in patients with relatively severe hypertension. But restricting screening to this population allows less severe and, possibly, early cases to go undetected for many years, if they are ever diagnosed.
Monticone et al.12 screened 1,672 patients referred to a hypertension center in Italy. Using relatively conservative thresholds for positive results on screening (aldosterone-renin ratio > 30 ng/dL per ng/mL/hour and a plasma aldosterone concentration > 10 ng/dL), confirmatory testing with intravenous saline suppression identified primary aldosteronism in 10% to 12% of patients who met the screening criteria. More importantly, 4% of patients with untreated hypertension (blood pressure 140–159/90–99 mm Hg) were also found to have primary aldosteronism. These patients did not meet any screening criteria and would not have been tested outside a research setting.
Other studies show that even in high-risk groups in which screening is recommended, rates of screening and eventual diagnosis remain unacceptably low.5,6
The limitations in screening are further confounded by the arbitrary aldosterone-renin ratio cutoffs used. As noted, the most widely accepted threshold for a positive screening test is an aldosterone-renin ratio of 30 ng/dL per ng/mL/hour or higher with a minimum plasma aldosterone concentration of 15 ng/dL or higher. However, multiple studies have repeatedly demonstrated higher detection rates when the criteria were more relaxed.11 In particular, in patients with low plasma renin activity (< 1 ng/mL/hour), plasma aldosterone concentration cutoffs of 9 ng/dL,2 or even as low as 6 ng/dL,13 have increased the number of cases detected.
A practice-changing study. The strongest evidence yet of the limitation of our screening test of choice comes from a study by Brown et al.7 The study cohort was derived from five study protocols across the United States representing a spectrum of blood pressure phenotypes ranging from normotension to resistant hypertension. A total of 1,846 patients were included in the study.
Blood pressure medications were withdrawn, except in patients with resistant hypertension, in whom medications aside from mineralocorticoid receptor antagonists and epithelial sodium channel inhibitors were continued. Then, all patients underwent oral salt loading for three days, after which plasma renin activity, plasma aldosterone concentration and 24-hour urinary aldosterone excretion were measured. The authors used a conservative threshold aldosterone excretion rate of 12 μg/24 hours to define “biochemically overt primary aldosteronism” in the context of high sodium balance and suppressed renin activity.
The rates of primary aldosteronism were high, reaching 11.3% in normotensive patients. In hypertensive patients, the rates were 15.7% in those with untreated stage 1 hypertension, 21.6% in those with untreated stage 2 and 22% in those with resistant hypertension. In the subset of patients with suppressed renin, the rates were even higher, reaching 51.6% in those with resistant hypertension.
However, these rates are perhaps not the most important takeaway from this study. What was remarkable was how poorly the currently accepted screening protocol performed. In patients with confirmed overt primary aldosteronism, the sensitivity (26.9%) and negative predictive value (31.8%) of an aldosterone-renin ratio greater than 30 were low. Even after relaxing the aldosterone-renin ratio cutoff to a more liberal 20, the sensitivity was a mere 42.3%. Importantly, in the patients with resistant hypertension and confirmed primary aldosteronism, 24.5% had a plasma aldosterone concentration less than 10 ng/dL and would have been missed had the standard screening criteria been used. The authors emphasized the insensitivity of a single aldosterone-renin ratio compared with a 24-hour urine collection, given the known pulsatile nature of aldosterone secretion and the unrecognized fact that adrenocorticotropic hormone is a secretagogue of aldosterone leading to diurnal fluctuations.7
It is now clear that the current aldosterone-renin ratio screening test and long-accepted cutoffs are far from being the right answer. In an editorial accompanying the above study, Dr. John Funder ultimately labels the spot measurement of plasma aldosterone concentration and aldosterone-renin ratio “not merely useless, but actually misleading.”14
The limitations in our current understanding do not stop here. The designation of “biochemically overt primary aldosteronism” relies yet again on arbitrary cutoffs for the confirmatory testing (generally accepted as an aldosterone excretion rate of 12 to 14 μg/24 hours in the case of the oral salt loading test).2 The issue with this definition is that even milder renin-independent aldosterone production that fails to meet these cutoffs might not be entirely benign.
The concept of “nonclassic” or “subclinical” primary aldosteronism has been proposed over the past few years, describing a milder phenotype of dysregulated renin-independent aldosterone production. Indeed, the risk of incident hypertension is reported to be higher in normotensive individuals with a high aldosterone-renin ratio and plasma aldosterone concentration values not meeting the criteria for primary aldosteronism.15 A follow-up study also showed that the rate of incident hypertension was high in participants with the lowest renin concentrations.16 Brown et al.17 reported similar outcomes in another cohort, in which suppressed renin activity (≤ 0.5 ng/mL/hour) was associated with significantly higher risk of incident hypertension among normotensive participants compared with those with unsuppressed renin. Importantly, incident hypertension correlated positively with the plasma aldosterone concentration even at levels considered to be in the normal range.
Interest in the low-renin profile is not new. In 1969, Woods et al.18 reported that hypertensive patients with the low-renin phenotype responded to adrenal blockade with significant decreases in blood pressure. The clinical significance of this suppressed renin phenotype is further highlighted in the PATHWAY-2 study,19 which showed that spironolactone was the most effective add-on medication in resistant hypertension. Importantly, primary aldosteronism was excluded by a specialist before patients entered this study. In a follow-up analysis,20 there was favorable blood pressure response to spironolactone when renin was suppressed, and the response correlated positively with plasma aldosterone concentration and aldosterone-renin ratio (even within the “normal” range).20 This analysis raises the question as to whether this population of patients with resistant hypertension included some with a milder form of primary aldosteronism who simply did not meet the current criteria for diagnosis of the disease.
In data presented at the American Society of Nephrology in 2019 (unpublished), we evaluated 1,142 patients with chronic kidney disease in whom renin and plasma aldosterone concentration values were obtained. After excluding patients with primary aldosteronism, patients with suppressed renin values had more resistant hypertension and faster decline in glomerular filtration rate than those with unsuppressed renin. We speculate that some of these patients with suppressed renin may actually have a milder form of primary aldosteronism.
Dysregulated aldosterone production is not just a cause of resistant hypertension. Even at levels below the currently accepted criteria for primary aldosteronism, it is associated with adverse cardiovascular, metabolic and kidney outcomes.17,21 In the study by Brown et al.,7 a clear continuum of renin-independent aldosterone production was observed in each of the four blood pressure categories (normotension, stage 1 hypertension, stage 2 hypertension and resistant hypertension) despite the high sodium balance. As expected, the magnitude of aldosterone production was progressively higher across the blood pressure spectrum. Importantly, a continuous relationship was observed between the magnitude of the renin-independent aldosterone production as well as signs of excess mineralocorticoid activity (systolic and diastolic blood pressure, serum potassium level and urinary potassium-to-sodium ratio).
Given the above, the authors call for reframing our terminology of primary aldosteronism and the arbitrary binary cutoffs to highlight the spectrum of this condition characterized by renin-independent aldosterone production — one that goes from a subclinical form where patients can be normotensive, to milder clinically significant phenotypes not meeting current diagnostic criteria, and all the way to an overt phenotype of severe hypertension and hypokalemia.11
Current screening practices likely capture only a small portion of the more severe phenotype of primary aldosteronism. Given the potential cardiometabolic and kidney implications of even the milder forms of primary aldosteronism, the current guidelines need to be reexamined. This is particularly important, as these risks are potentially modifiable with targeted therapy with mineralocorticoid receptor antagonists. In Figure 1, we propose an approach to screening and diagnosing primary aldosteronism.
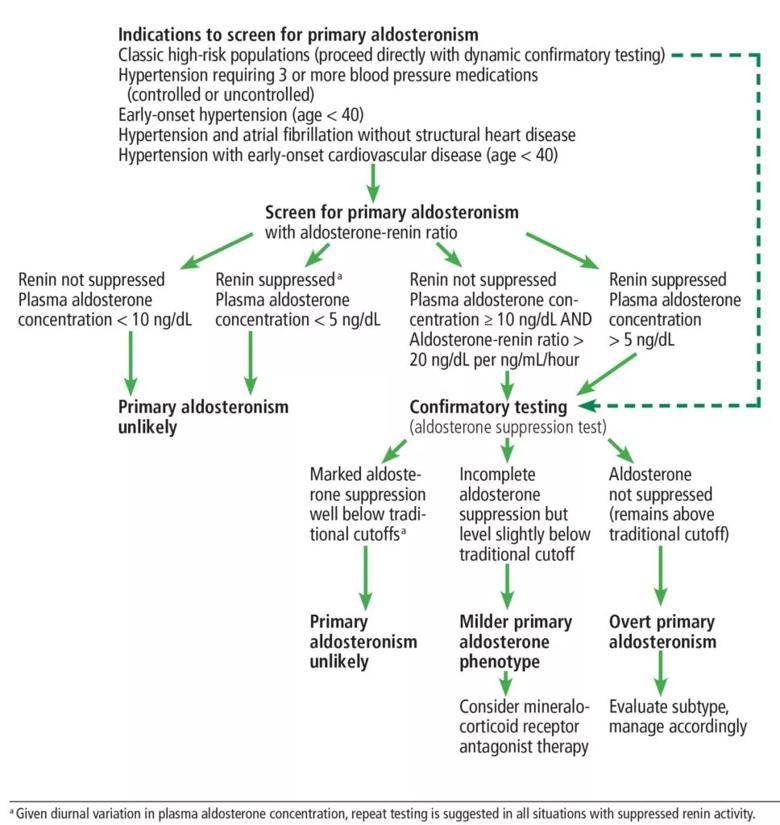
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/f9928309-9b52-4f43-9fdd-55c88d98835c/F1_large_-1-968x1024_jpg)
Figure 1. Our suggested algorithm for screening for and diagnosis of primary aldosteronism.
New practice guidelines hopefully will address the following issues:
Our understanding of primary aldosteronism has changed dramatically over the past few years. Formerly seen as a rare syndrome of resistant hypertension and hypokalemia, primary aldosteronism should now be viewed as a spectrum of autonomous renin-independent aldosterone production that is prevalent across the continuum of blood pressure severity. This condition is associated with higher cardiovascular, metabolic and renal morbidity, even in its milder phenotypes.
Current screening and diagnostic guidelines capture only a fraction of the more severe forms of primary aldosteronism and with very poor overall sensitivity. A revamping of current practice guidelines is needed, informed by our newer understanding of this disorder.
Drs. Mehdi and Thomas are staff in Cleveland Clinic’s Department of Nephrology and Hypertension. Dr. Rao is Quality Improvement Officer in the Endocrinology and Metabolism Institute.
References are available here, in the Cleveland Clinic Journal of Medicine version of this article.
Advertisement

Pheochromocytoma case underscores the value in considering atypical presentations

Advocacy group underscores need for multidisciplinary expertise

A reconcilable divorce

A review of the latest evidence about purported side effects

High-volume surgery center can make a difference

Advancements in equipment and technology drive the use of HCL therapy for pregnant women with T1D

Patients spent less time in the hospital and no tumors were missed

A new study shows that an AI-enabled bundled system of sensors and coaching reduced A1C with fewer medications