Reproducible technique uses native recipient tissue, avoiding risks of complex baffles
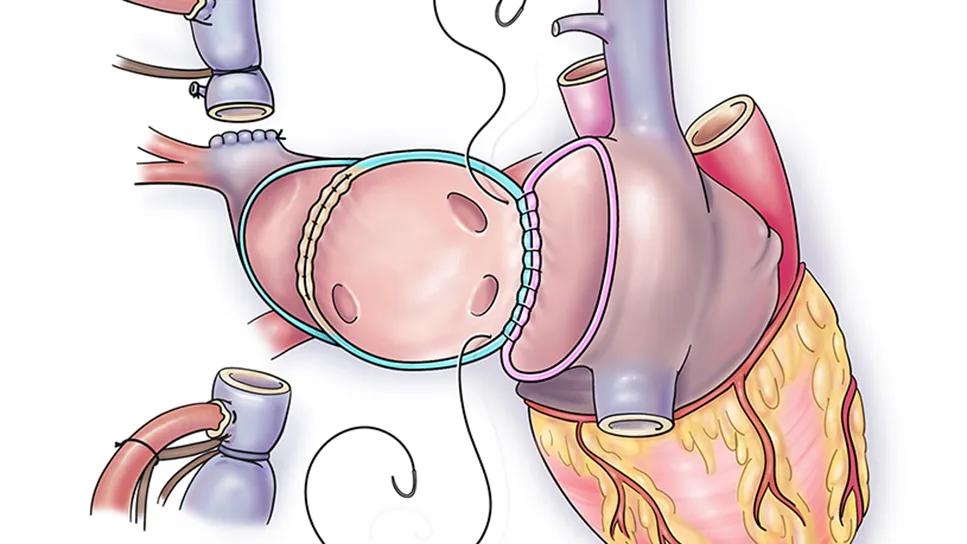
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/35d9e70e-3c85-46be-84ce-9829fad362e4/Heart-Transplant-with-Anomalous-RUPV-feature)
illustration of a heart operation
Cleveland Clinic cardiac surgeons have pioneered an innovative approach to repair of a partial anomalous pulmonary venous return (PAPVR) at the time of heart transplantation and using native recipient tissue.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The technique, described in a recent case series in The Journal of Heart and Lung Transplantation (2025;44[7]:1189-1191), offers a reproducible solution that preserves right ventricular function while avoiding risks associated with complications in other repair methods.
“For heart transplant candidates with PAPVR, correction of intracardiac shunts is essential to preserve the implanted heart’s ventricular function and optimize long-term outcomes,” explains Professor Hani Najm, MD, MSc, Chair of Pediatric and Congenital Heart Surgery and senior author of the case series report. “This is especially crucial in adults with congenital heart disease, in view of their lifetime hemodynamic burden and predisposition to elevated pulmonary pressures.”
While PAPVR has an estimated prevalence of 0.5% in the general population, it is more common in patients with complex congenital heart disease, in whom the prevalence may be as high as 22%.
PAPVR typically results in inappropriate shunting of oxygenated blood to the right atrium. Patients with a clinically significant PAPVR may require surgical correction, typically the Warden procedure, which involves intracardiac baffles that carry the risk of superior vena cava obstruction (SVC) and other complications.
The only previous report of PAPVR repair concomitant with heart transplant involved a variation of the Warden procedure using pericardial patches, which may lead to calcification and pulmonary venous stenosis. This limitation prompted Dr. Najm and colleagues to develop their novel approach to concomitant repair using native recipient tissue.
Advertisement
The surgical team reported their experience with two distinct cases that demonstrate the versatility of their technique:
The technique involves several critical steps that differentiate it from other approaches:
Extended organ procurement: The procurement team recovers the donor organ with SVC length extended to include the innominate vein, providing adequate tissue for reconstruction.
Careful recipient preparation: Bicaval cannulation is performed high at the innominate vein junction, preserving adequate length for reconstruction while carefully dissecting all anomalous pulmonary veins.
Novel anatomical reconstruction: After cardiectomy, the SVC is divided above the pulmonary veins, after which the cardiac end of the SVC is oversewn. A “neo-left atrium” is created by resecting the atrial septum of the recipient heart; as the heart is removed for transplantation, the SVC remains attached to the left atrium posteriorly. The left atrial cuff is then anastomosed to the donor left atrial cuff, allowing the pulmonary veins to drain to the left atrium (Figure).
Advertisement
Native tissue utilization: The technique uses exclusively native recipient tissue, avoiding synthetic materials or pericardial patches that could lead to long-term complications.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/c45626a8-5bc6-438b-a06f-53b2999a3722/Heart-Transplant-with-Anomalous-RUPV)
Figure. Key steps in the technique. (A) Division of the SVC above the location of the pulmonary veins. The proximal SVC is oversewn with running suture fashion. (B) The donor atrial cuff is anastomosed to the enlarged neo-left atrium recipient cuff. (C) The final result showing anomalous pulmonary veins entering the left atrium and the long SVC anastomosis close to the recipient innominate vein. Reprinted from Potz BA, et al. J Heart Lung Transplant. 2025;44[7]:1189-1191. Illustrations © Cleveland Clinic.
In both cases, postoperative transesophageal echocardiography demonstrated unrestricted pulmonary flow through the anomalous pulmonary veins to the neo-left atrium, with good biventricular function.
“The technique successfully redirected anomalous venous return to the systemic circulation while maintaining optimal hemodynamics,” Dr. Najm observes.
He and his co-authors identify several advantages of this approach to managing PAPVR in heart transplant candidates:
“This novel native tissue technique elegantly redirects anomalous pulmonary venous flow to the transplanted left atrium while avoiding the complexity and risks of intracardiac baffles,” notes co-author Anthony Zaki, MD, a Cleveland Clinic cardiac surgeon whose specialty interests include heart transplant. “By providing a standardized, reproducible approach, it promises a durable solution that addresses the limitations of previous approaches to PAPVR repair.”
Advertisement
Advertisement
Innovative hardware and AI algorithms aim to detect cardiovascular decline sooner
Experts advise thorough assessment of right ventricle and reinforcement of tricuspid valve
A reliable and reproducible alternative to conventional reimplantation and coronary unroofing
Program will support family-centered congenital heart disease care and staff educational opportunities
Case provides proof of concept, prevents need for future heart transplant
Pre and post-surgical CEEG in infants undergoing congenital heart surgery offers the potential for minimizing long-term neurodevelopmental injury
Science advisory examines challenges, ethical considerations and future directions
Updated guidance and a call to action