Getting patients to their goal blood pressure
By Jordana Yahr, DO; George Thomas, MD; Juan Calle, MD; Jonathan J. Taliercio, DO
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Note: This article is reprinted from the Cleveland Clinic Journal of Medicine without references (2023;90[2]:115-125).
Most patients with hypertension do not achieve current recommended blood pressure targets. It is imperative that physicians recognize risk factors associated with resistant hypertension in order to better control it. In this article, we discuss the epidemiology, diagnosis, evaluation, and treatment of resistant hypertension and a stepwise approach (Figure 1) to getting patients to their goal blood pressure.
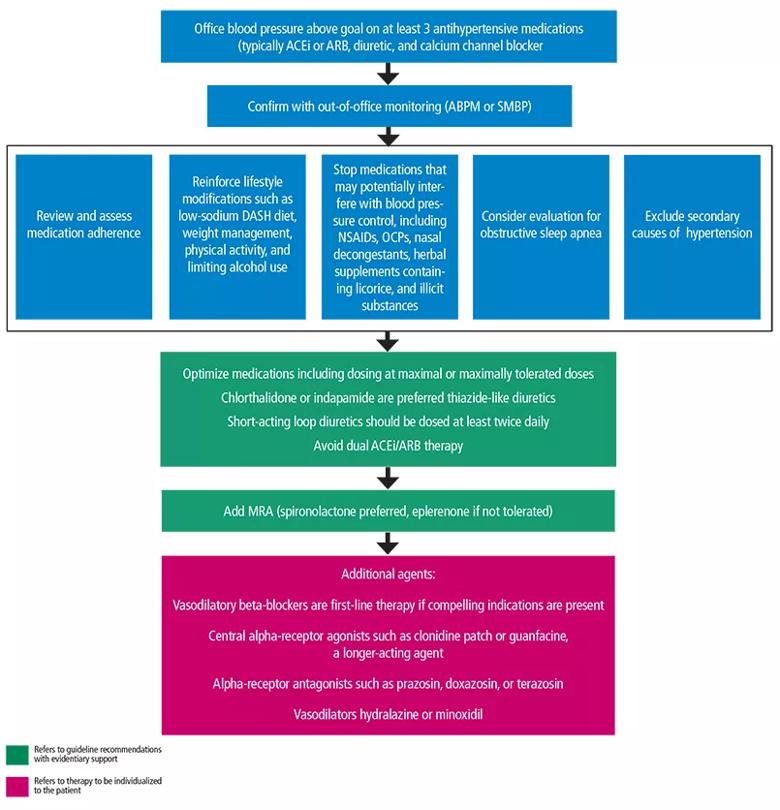
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/06d9579e-0a64-4aaf-a49e-acb1808af0f3/23-URL-3883960-CQD-Inset-1_jpg)
Figure 1. Management of resistant hypertension, recommendations adapted from the American Heart Association scientific statement on resistant hypertension.
The 2017 American College of Cardiology (ACC) and American Heart Association (AHA) guidelines define hypertension as systolic blood pressure 130 mm Hg or higher or diastolic blood pressure 80 mm Hg or higher, based on at least two readings obtained on at least two occasions. This is stricter than the 2003 guidelines from the Seventh Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure, which defined hypertension as blood pressure 140/90 mm Hg or higher.
As a result of the new definition, the prevalence of hypertension in the United States increased from roughly 32% to 47%. Hypertension is a leading cause of cardiovascular disease and death. Its management costs the US healthcare system approximately $131 billion annually.
Blood pressure targets should be individualized based on patient characteristics, medication side effects, patient tolerance and preferences. In patients with cardiovascular disease or with a risk of an atherosclerotic cardiovascular disease event of 10% or higher in the next 10 years, the 2017 ACC-AHA guidelines say that a goal of less than 130/80 mm Hg “is recommended.”
Advertisement
In patients at lower risk, the ACC-AHA guidelines say the same goal “may be reasonable.”
In patients with chronic kidney disease, the 2021 Kidney Disease Improving Global Outcomes guidelines recommended keeping the systolic blood pressure lower than 120 mm Hg contingent on proper blood pressure measurement. This recommendation is based largely on the cardiovascular benefits of this lower goal demonstrated in the Systolic Blood Pressure Intervention Trial, in which patients at risk of cardiovascular disease but without diabetes were randomized to goal blood pressures of either less than 120 mm Hg or less than 140 mm Hg.
In a chronic kidney disease subgroup analysis, the intensive group had a slightly higher rate of change in estimated glomerular filtration rate (−0.47 vs −0.32 mL/min/1.73 m2 per year; P < .03) after six months. The decline in kidney function may be hemodynamically mediated as a result of more intensive blood pressure control.
In patients with diabetes, the American Diabetes Association recommends a target blood pressure lower than 130/80 mm Hg.
Most people are not meeting these goals. According to an estimate from the US Centers for Disease Control and Prevention, of the 116 million Americans with hypertension, only 23.9 million (20.6%) have their blood pressure controlled using the 2017 ACC-AHA definitions. The control rate was 62.8% using the old threshold of less than 140/90 mm Hg.
Resistant hypertension is defined by the ACC-AHA as blood pressure that is above goal despite the patient receiving at least three medications with different mechanisms of action. All medications must be prescribed at maximally tolerated doses and should preferably include a long-acting dihydropyridine calcium channel blocker, either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB), and a diuretic. Resistant hypertension is also defined as controlled blood pressure on at least four antihypertensive medications.
Advertisement
Pseudoresistance is suboptimal blood pressure control secondary to medication nonadherence, white coat effect, or poor measurement technique.
Apparent treatment-resistant hypertension is the term used in epidemiologic studies to refer to cases in which patients meet the criteria for resistant hypertension but have unverified adherence or medication dosing or have not undergone out-of-office blood pressure monitoring to rule out the white-coat effect.
The prevalence of resistant hypertension is difficult to ascertain precisely, given the need to rule out pseudoresistance. However, an estimate from the National Health and Nutrition Examination Survey (NHANES) put the prevalence of apparent treatment-resistant hypertension (using the cutoff of ≥ 140/90 mm Hg) in the general public at 12.8%. In hypertensive patients in the Chronic Renal Insufficiency Cohort, the prevalence of apparent treatment-resistant hypertension using the same definition was 40.4%. Other comorbidities associated with resistant hypertension include older age, obesity, diabetes mellitus, and obstructive sleep apnea.
Resistant hypertension is associated with worse outcomes, particularly adverse kidney outcomes and cardiovascular morbidity and death. In a study of 10,001 patients, apparent treatment-resistant hypertension was associated with a 64% higher incidence of the composite cardiovascular outcome of fatal coronary heart disease, nonfatal myocardial infarction, cardiac arrest, and stroke. Apparent treatment-resistant hypertension was shown to increase the risk of kidney failure in an analysis of participants from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (hazard ratio 1.95; 95% confidence interval 1.11–3.41).
Advertisement
The diagnosis of resistant hypertension requires ruling out pseudoresistance due to medication nonadherence, improper blood pressure measurement, and the white-coat effect.
Is the patient taking the medication?
Medication nonadherence is a discrepancy between how a medication is prescribed and how the patient is actually taking it. Its prevalence in patients with apparent treatment-resistant hypertension is difficult to determine. Studies have shown it ranging from 3% to 86%, with a pooled estimate of 31% in a meta-analysis.
Medication adherence can also be difficult to address, but several techniques have been studied. Some laboratories offer serum and urine assays to detect metabolites of antihypertensive drugs, and pharmacy-based assessments include pharmacy fill history and pill counting. Directly observed therapy has been shown to reduce resistant hypertension by 29%. Each of these methods has limitations such as inaccuracies in patient reporting and physician interviewing, as well as the impracticality and cost of directly observing therapy or measuring drug metabolites.
Ensuring that patients understand their medication instructions and involving them in shared decision-making are important to improve adherence.
Are the measurements accurate?
Measuring blood pressure accurately requires proper technique, proper cuff size, and use of validated devices (Table 1). Automated office blood pressure monitoring devices are favored over conventional manual auscultatory devices for office use. These devices are designed to take multiple blood pressure readings in one sitting. In one study, the mean systolic blood pressure taken by automated office blood pressure devices was 11 mm Hg lower than those obtained with manual in-office devices, and the results from in-office automated devices were more consistent with those of ambulatory blood pressure monitoring.
Advertisement
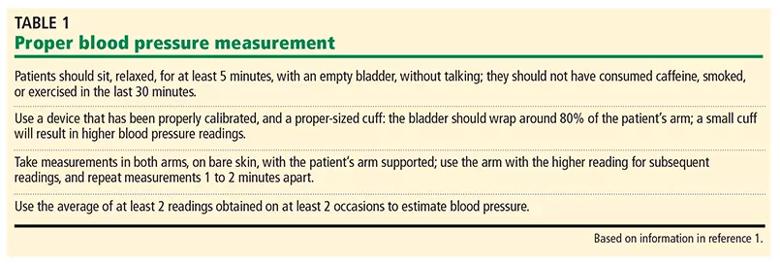
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2e4f050e-65ca-4d48-a1dc-b3c1e0304aba/23-URL-3883960-CQD-Inset-2_jpg)
Table 1. Based on information in reference 1.
Sources of inaccuracy with auscultatory blood pressure measurement include inadequate operator skill, inability to hear Korotkoff sounds, and terminal digit bias. If you measure blood pressure using the auscultatory technique, you should pay careful attention to operator training, proper cuff size, and technique. Aneroid sphygmomanometers require more frequent calibration than oscillatory machines.
What is the patient’s blood pressure out of the office?
Current guidelines recommend measuring blood pressure out of the office to complement in-office measurement to control hypertension, but not as the sole measurement. It can improve diagnostic accuracy and help detect other forms of hypertension such as white-coat or masked hypertension (see discussion below). Two monitoring methods are used: ambulatory and self-measured.
Self-measurement means the patient takes their blood pressure at regular times during the day. While there is no consensus on the optimal schedule for checking blood pressure at home, two to three consecutive measurements can be performed twice daily in the morning and evening, for a minimum of three and, ideally, five five to seven consecutive days every month. We recommend measuring blood pressure before taking antihypertensive medications to better assess control.
Ambulatory monitoring records blood pressure over a 24-hour period. An advantage is its ability to measure nocturnal blood pressure. Blood pressure normally dips by 10% to 20% during sleep, and patients who are “nondippers” are at higher risk of cardiovascular events.
White-coat hypertension is elevated in-office blood pressure readings with normal out-of-office blood pressure in a person not being treated with antihypertensive medication (Table 2). In contrast, the white-coat effect is the same pattern in a person who is receiving treatment for hypertension. The white-coat effect may be seen in 28% to 39% of those with resistant hypertension. Untreated white-coat hypertension is associated with a higher risk of cardiovascular events compared with sustained normotension.
In contrast, in a recent meta-analysis, patients with the white-coat effect (i.e., on treatment, with normal blood pressures at home but high blood pressure in the office) showed no increase in cardiovascular risk compared with those with controlled hypertension.
Masked hypertension is normal office blood pressure readings but elevated out-of-office readings. Patients with masked hypertension are at higher risk of cardiovascular events than normotensive patients or those with white-coat hypertension.
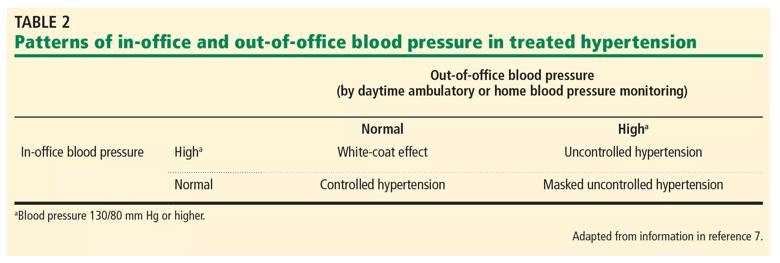
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/a3da6093-f4af-43d2-9caa-0cae6d1935ef/23-URL-3883960-CQD-Inset-3_jpg)
Table 2. a = Blood pressure 130/80 mm Hg or higher.
Does the patient have lifestyle factors that raise blood pressure?
Obesity
The relationship between increased adiposity and elevated blood pressure has been well established. NHANES participants who had a body mass index of 30 kg/m2 or higher were twice as likely to have resistant or apparent treatment-resistant hypertension.
Pathogenic mechanisms of obesity-related hypertension include increased salt sensitivity, increased sympathetic nervous system activity, activation of the renin-angiotensin-aldosterone system, and aldosterone secretion by adipose tissue. Of these mechanisms, aldosterone secretion by adipose tissue is the only one that is obesity-specific, as the others can also occur in diseases such as chronic kidney disease and heart failure.
In hypertensive adults in NHANES, ACE inhibitors and ARBs had a more pronounced antihypertensive effect in women with obesity than in women without obesity. This effect was not seen in men. However, there are currently no blood pressure guidelines that have specific medication recommendations for patients with obesity vs nonobesity.
The amount of sodium in the diet
Dietary sodium increases blood pressure. This effect may not occur in all people, but certain groups are more salt-sensitive, including older adults and patients with chronic kidney disease. In a randomized crossover trial in 12 patients with resistant hypertension, reducing dietary sodium from 250 mmol/day (5,750 mg) per day for 1 week to 50 mmol (1,150 mg) per day for 1 week lowered office systolic blood pressure by 22.7 mm Hg (95% confidence interval –33.5 to –11.8; P = .008).
Patients with resistant hypertension had more significant blood pressure reductions than other patients with hypertension or the general population, suggesting salt sensitivity may play a bigger role in the pathogenesis of resistant hypertension and reinforcing the importance of including a diuretic in the treatment plan. Patients should be counseled to adhere to a diet with less than 2 g of sodium per day (5 g of table salt) in addition to the DASH (Dietary Approaches to Stop Hypertension) diet, which is low in sodium and rich in fruit, vegetables, and low-fat dairy products, as the combination of these two was shown to be more effective than either alone.
Recommended exercise
Aerobic exercise has been shown to reduce blood pressure in patients with hypertension and resistant hypertension. Patients with resistant hypertension who enrolled in a treadmill exercise program of 8 to 12 weeks lowered their daytime systolic ambulatory blood pressure by 5.9 mm Hg (± 11.6 mm Hg; P = .03). In another study, those who exercised for 60 minutes in a heated pool three times per week for two weeks experienced a reduction of 12 mm Hg in 24-hour ambulatory systolic blood pressure and a reduction of 9 mm Hg in diastolic blood pressure.
Alcohol consumption
Regular alcohol consumption has been shown to increase blood pressure by 1 mm Hg for every 10 g of alcohol consumed (approximately 1 standard drink), an effect that is reversible within a few weeks of cessation.
Smoking, chewing, vaping
Nicotine, most commonly contained in cigarettes, vaping fluid, and smokeless tobacco, causes an acute rise in blood pressure. Cessation should be recommended to all patients in general, and especially to those with resistant hypertension to ameliorate their already increased risk of cardiovascular events.
Is the patient taking medications that raise blood pressure?
Medications that can raise blood pressure include the following:
The degree of blood pressure effect from these medications may vary widely from person-to-person.
Patients with resistant hypertension should be evaluated for secondary hypertension, since recognition and directed therapy may improve blood pressure control. In this section, we discuss common causes of secondary hypertension, the clinical context in which they should be suspected, and the basic screening for each.
Kidney parenchymal disease
Hypertension is both a cause and a consequence of chronic kidney disease and is common in this patient population. Of 3,612 patients participating in the Chronic Renal Insufficiency Cohort study, 85.7% had a diagnosis of hypertension at their baseline visit. Fewer than half (46.1%) had their blood pressure lower than 130/80 mm Hg.
Proposed mechanisms of hypertension in kidney disease include an upregulated renin-angiotensinaldosterone system, increased salt and fluid retention, endothelial dysfunction, and increased sympathetic nervous system activity.
Kidney disease should be assessed and considered as a risk factor for resistant hypertension in patients with an elevated serum creatinine or abnormal urinalysis.
Primary aldosteronism
Primary aldosteronism (i.e., hyperaldosteronism) is due to autonomous hypersecretion of aldosterone. Excess circulating aldosterone leads to salt and water retention and renal potassium wasting, which results in hypertension and cardiovascular disease.
Primary aldosteronism is more common than previously thought and often goes undiagnosed, with a prevalence ranging from 8% to 30% in various hypertensive populations. Hypokalemia as a result of renal potassium wasting is present in only 9% to 37% of patients who have primary aldosteronism, so this disease can be underrecognized.
Measuring the plasma aldosterone-to-renin ratio is the test most often used to screen for primary aldosteronism. However, this test has the potential for false-positive and false-negative results, depending on whether patients are taking medications that interfere with the renin-angiotensin-aldosterone system, the cutoff values used, the time of testing, and the body positioning at the time of testing (morning preferred, after being seated for 15 minutes).
The Endocrine Society guidelines recommend initial testing with the aldosterone-renin ratio followed by a confirmatory test (intravenous or oral salt-loading test) for patients with hypertension who are at risk of primary aldosteronism. Patients at risk include those with resistant hypertension on optimal therapy, those with hypertension with spontaneous or diuretic-induced hypokalemia, and those with hypertension with adrenal incidentaloma, as well as hypertensive first-degree relatives of patients with primary aldosteronism.
An aldosterone-renin ratio of 20 or higher should warrant further investigation if the plasma aldosterone concentration is 15 ng/dL or higher. Patients with very low renin levels, spontaneous hypokalemia, and a plasma aldosterone concentration higher than 20 ng/dL likely do not require confirmatory testing and should move forward with adrenal imaging. Primary aldosteronism is treated with surgery if a unilateral aldosterone-secreting adenoma is found, or is treated with mineralocorticoid receptor antagonists such as spironolactone or eplerenone in bilateral ad renal disease and in patients who are not candidates for surgery.
A full discussion of primary aldosteronism is beyond the scope of this article, but screening and diagnosis according to current guidelines may detect only a fraction of patients with primary aldosteronism, and a revamping of current practice guidelines is needed.
Obstructive sleep apnea
Obstructive sleep apnea is very common in patients with resistant hypertension. Proposed mechanisms by which it could cause or worsen hypertension include increased upper-airway resistance, hypoxia, and hypercapnia. These cause endothelial reactivity, inflammation, oxidative stress, and increased sympathetic and renin-angiotensin-aldosterone system activity, which ultimately lead to increased vascular tone and hypertension.
Treating obstructive sleep apnea with continuous positive airway pressure (CPAP) in patients with resistant hypertension has been shown to decrease 24-hour ambulatory blood pressure, and the more hours per night that patients actually use it, the greater the effect on blood pressure. However, although treating obstructive sleep apnea with CPAP is recommended to reduce the risk of other cardiovascular complications, a meta-analysis found only a modest reduction of 2.46 mm Hg in systolic blood pressure.
Obesity and obstructive sleep apnea are both risk factors for resistant hypertension, but a study that looked at the effect of CPAP therapy on blood pressure in patients with obesity vs those without obesity found no significant difference between the groups.
Given the high prevalence of obstructive sleep apnea in those with resistant hypertension, screening for it should be common in this population. Screening tools such as the STOP-BANG score can help risk-stratify patients who have suggestive symptoms and who should be tested with polysomnography, the gold standard for diagnosis.
(STOP-BANG consists of eight factors, which spell the acronym: snoring, tired or sleepy during the day, observed stopping breathing while sleeping, high blood pressure, body mass index higher than 35 kg/m2, age older than 50, neck circumference ≥ 17 inches if a man or ≥ 16 inches if a woman, and male sex. If three or more factors are present, the patient has a high risk of obstructive sleep apnea.)
Renovascular hypertension
Renovascular hypertension is a syndrome of elevated blood pressure due to diminished renal arterial blood flow resulting in kidney ischemia. It is most commonly caused by atherosclerosis of the renal arteries, but other pathologic processes include fibromuscular dysplasia, renal artery infarct or dissection, and vasculitis.
The diagnosis of renal artery stenosis includes imaging with duplex ultrasonography, computed tomography angiography, or magnetic resonance angiography. At least 70% of the renal artery must be stenosed before the lesion can be considered to be causing the hypertension.
Atherosclerotic renovascular disease is considered a coronary artery disease equivalent, and its treatment consists of medical management focused on blood pressure, lipid and glucose control, and antiplatelet therapy. Percutaneous revascularization should generally be considered in patients with the following high-risk features:
Other endocrinopathies
Catecholamine-secreting tumors such as pheochromocytomas and paragangliomas are rare causes of hypertension, accounting for 0.2% to 0.6% of cases, but are associated with significant mortality risk. Symptoms that should prompt screening include paroxysmal headaches, diaphoresis, and tachycardia.
The 2014 Endocrine Society guidelines recommend screening by measuring either plasma-free metanephrines or 24-hour urine fractionated metanephrines. Patients who have plasma metanephrines measured should lie supine for at least 30 minutes before sampling. Normetanephrine and metanephrine levels 3 or more times higher than the upper limit of normal are highly suggestive of a catecholamine-producing tumor.
Medications that can lead to elevated levels of metanephrines and catecholamines include tricyclic antidepressants, amphetamines, monoamine oxidase inhibitors, and levodopa, and withdrawal from clonidine can have the same effect.
Cushing disease or syndrome (hypercortisolism from glucocorticoid excess) is a relatively uncommon cause of resistant hypertension. Cushing syndrome is a constellation of symptoms that classically include glucose intolerance, acne, osteoporosis, obesity, menstrual changes, hirsutism, muscle wasting, and moon facies.
Interestingly, in one study, 26.5% of patients with resistant hypertension but no overt signs and symptoms of Cushing syndrome had biochemical evidence of hypercortisolism, suggesting that clinicians should consider testing for it in patients without the classic syndrome. Patients should be screened by measuring the 24-hour urine cortisol level or late-night salivary cortisol level, or by a low-dose dexamethasone suppression test.
Less common endocrine disorders that can contribute to resistant hypertension include disorders of the thyroid and parathyroid glands. Thyroid-stimulating hormone should be checked in those with difficult-to-control hypertension. Testing for primary hyperparathyroidism should be considered in any patient presenting with hypercalcemia.
All patients diagnosed with resistant hypertension should be screened for causes of secondary hypertension based on history, physical findings, and individual risk factors. A multifactorial approach to treat resistant hypertension includes a combination of lifestyle modification, pharmacotherapy, and addressing underlying contributing diseases.
Patients with resistant hypertension should be screened for end-organ damage — e.g., with serum creatinine and urinalysis to look for kidney disease, electrocardiography or echocardiography to assess for left ventricular hypertrophy, and an ophthalmologic examination to look for hypertensive retinopathy.
Pharmacologic therapy
Prescribing antihypertensive therapy begins with identifying comorbidities that require first-line agents that have a compelling indication, such as beta-blockers for heart failure, history of myocardial infarction, or aortic dissection, or drugs that block the renin- angiotensin-aldosterone system for proteinuria. The initial pharmacologic approach to resistant hypertension consists of three medications, each mechanistically different, at maximally tolerated doses, as follows:
In patients with preserved glomerular filtration rate, the preferred first-line diuretic is either chlorthalidone or indapamide because of their longer half-life and more potent antihypertensive effect compared with hydrochlorothiazide. Loop diuretics are preferred in patients with an estimated glomerular filtration rate less than 30 mL/min/1.73 m2. Torsemide can be used once a day, but shorter-acting loop diuretics such as furosemide or bumetanide must be dosed at least twice a day. A recent randomized controlled trial showed that chlorthalidone was effective in those with an estimated glomerular filtration rate of 15 to 30 mL/min/1.73 m2, thus representing another available agent in this population.
If blood pressure is still not controlled on maximally tolerated therapy with these three agents, a mineralocorticoid receptor antagonist (spironolactone or eplerenone) should be the fourth-line agent. The PATHWAY-2 trial demonstrated that spironolactone was superior in reducing blood pressure compared with bisoprolol (a beta-blocker), doxazosin (an alpha-blocker), or placebo as add-on therapy in patients with resistant hypertension on three blood pressure medications.
Side effects of spironolactone include hyperkalemia and gynecomastia, and the drug should be used with caution in chronic kidney disease. If gynecomastia becomes intolerable, spironolactone can be switched to eplerenone, a selective aldosterone receptor antagonist that has minimal interaction with sex hormone steroid receptors. However, spironolactone is preferred since it has been extensively studied, costs less, and requires only daily dosing because of its longer half-life compared with eplerenone.
The addition of other agents should be based on individual factors. Vasodilating beta-blockers (labetalol, carvedilol, nebivolol, bisoprolol) may be the preferred fifth-line agent. Other choices include clonidine, a centrally acting alpha-2 agonist. Clonidine can be given as a transdermal patch to improve adherence, minimize frequent oral dosing, and lower the risk of rebound hypertension.
According to the AHA guidelines, if blood pressure is still not at goal, hydralazine may be initiated at a starting dose of 25 mg 3 times a day, with the addition of a nitrate in the presence of heart failure with reduced ejection fraction. Finally, minoxidil may be used if hydralazine is not tolerated. Hydralazine and minoxidil are associated with fluid retention and reflex tachycardia.
Recent studies have shown that aldosterone synthase inhibitors and dual endothelin antagonists may be effective in resistant hypertension. While neither are approved at this time by the U.S. Food and Drug Administration (FDA) for this indication, these agents may represent additional treatment options upon further study.
Patients should be referred to a hypertension specialist if blood pressure remains uncontrolled despite the above therapies.
Experimental devices and other therapies are currently being explored in patients with resistant hypertension. Renal denervation to blunt sympathetic tone showed no benefit in the Renal Denervation in Patients With Uncontrolled Hypertension (SYMPLICITY HTN-3) study. The Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN TRIO), utilizing a newer catheter design and a stricter medication protocol, demonstrated a decrease of 5.8 mm Hg compared with controls, a modest benefit.
Other experimental therapies aimed at sympathetic tone modulation include carotid baroreceptor activation therapy and carotid baroreceptor amplification therapy. None of these device therapies are currently FDA-approved, and more studies are needed to determine their long-term efficacy and safety.
Advertisement

Phase 2b trial of aldosterone synthase inhibitor also finds no benefit from dose escalation

Fixed-dose single-pill combinations and future therapies

Clinicians should individualize dosing practices based on patient risk factors and preferences
Insights for diagnosing, assessing and treating
General principles for use of the long-awaited new therapy approach

Results inform dose selection for further trials of the aldosterone synthase inhibitor

Pediatric urologists lead quality improvement initiative, author systemwide guideline

Reproductive urologists publish a contemporary review to guide practice