Study also finds that 26% of children with cancer have mutations in DNA repair genes
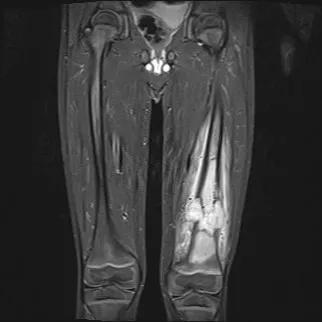
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2edc3a28-ccc8-4433-842b-f18f2561bb31/Picture1-jpg)
Osteosarcoma
Researchers have discovered a gene that predisposes patients to osteosarcoma, the bone tumor with the worst prognosis in children and adolescents. The SMARCAL1 gene was among nine genes associated with various cancers in a new study, the first comprehensive assessment of DNA repair genes in pediatric cancers.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
While past studies have explored pathogenic variants in approximately 100 cancer-predisposing genes, this is the first time that pathogenic signals were comprehensively investigated across genes in DNA repair pathways.
“Our study revealed that heritable variants in DNA repair genes are common among children with cancer,” says senior author Richa Sharma, MD, a pediatric hematologist and oncologist at Cleveland Clinic. “Of the 5,993 children with cancer that we studied, 26% had at least one variant in a DNA repair gene.”
Dr. Sharma and a multicenter team of researchers recently published their discovery in the Journal of Clinical Oncology.
Why children get cancer has puzzled Dr. Sharma since medical school.
“It’s understandable that adults get cancer, as mutations due to environmental exposures develop over time,” she says. “But children around the world, sometimes as young as days old, don’t have enough time to accumulate the mutational burden to get aggressive cancers, yet are diagnosed with cancer. So, why does a 1-year-old get acute myeloid leukemia? Why does an 11-year-old get a metastatic bone tumor? With my background in basic biology, I thought there must be something predisposing them genetically — maybe heritable deficiencies in DNA repair pathways, which when healthy can function as tumor suppressors.”
During her time at St. Jude Children’s Research Hospital and after joining Cleveland Clinic in 2025, Dr. Sharma worked with her multicenter team to look for answers in a novel way. Instead of screening a patient population for known cancer-predisposition genes, she “went biology first,” studying conventionally known DNA repair pathways. Specifically, she and collaborators at St. Jude searched for 189 DNA repair genes in the germline exomes of nearly 6,000 children with cancer (median age 6) and more than 14,000 adults without cancer.
Advertisement
Sequencing data from 20,000 controls and cancer patients was a herculean task undertaken by bioinformatics experts, Dr. Sharma says, but the resulting database was the muscle that powered the research team’s big discovery: hits on mutations in nine specific genes in seven specific cancers.
Germline predisposing variants in five genes identified in the study had previously been associated with cancers:
Four more associations were newly identified:
“Finding the previously known associations helped validate that our analytic bioinformatics pipeline was working,” Dr. Sharma says. “Because we could confirm the link between germline TP53 mutation in high-grade glioma, for example, we could be confident in the novel signals we were finding.”
Of the four genes newly associated with cancers in the discovery cohort, only one validated in all three replication cohorts: SMARCAL1 in osteosarcoma (16/627, 2.6%). BRCA1 in ependymoma and SMC5 in medulloblastoma each validated in one replication cohort.
“SMARCAL1 is definitely a cancer predisposition gene for osteosarcoma, but that’s just the beginning of the revelations that we hope to make as we dive deeper into its biologic mechanisms,” Dr. Sharma says.
Advertisement
Based on this evidence, Dr. Sharma promotes adding SMARCAL1 to the genetic panel used when performing genetic surveillance for families and patients with osteosarcoma. It could lead to earlier detection of disease.
Just as exciting, she says, is using SMARCAL1 as a target for future therapies for osteosarcoma and other cancers. Mutations in the gene have been implicated in other sarcomas and high-grade brain tumors including glioblastoma.
“Although there’s a difference between inheriting versus acquiring a mutation in a gene, the fact that there is a mutation present, in this case in SMARCAL1, implies its importance for tumor biology,” Dr. Sharma says. “By studying the biology of SMARCAL1 and how it drives tumor growth, whether it be from inheriting or acquiring the mutation, we could potentially explore ways of cutting off its oncogenic potential.”
That’s the next step in Dr. Sharma’s research. Her team’s recent study suggests a starting point: All four SMARCAL1-associated osteosarcoma tumors in the replication cohorts had alternative-lengthening of telomeres (ALT). Could ALT be the mechanism encouraged by SMARCAL1 dysfunction, thereby driving growth of osteosarcoma?
“My lab focuses on DNA repair and telomere biology, and SMARCAL1 happens to straddle those two areas,” she says. “This opportunity just landed in my lap. We need to explore it. Yes, discovering that SMARCAL1 dysfunction promotes osteosarcoma is important, but the big deal will be when we uncover how and why.”
Advertisement
Advertisement
Research highlights promising outcomes for treating recurrent and metastatic cases
Biologic approaches, growing implants and more
Case study of radial-to-axillary nerve transfer for tumor-related deltoid nerve injury
Tapping into motivational interviewing to guide behavioral change
Complex disease requires a comprehensive approach
Rare genetic disorder prevents bone mineralization
Self-efficacy mindset, burst therapy and increased biofeedback may help improve outcomes
6 objective measures to assess pain and evaluate progress