How Cleveland Clinic approaches the condition in the absence of randomized trial data
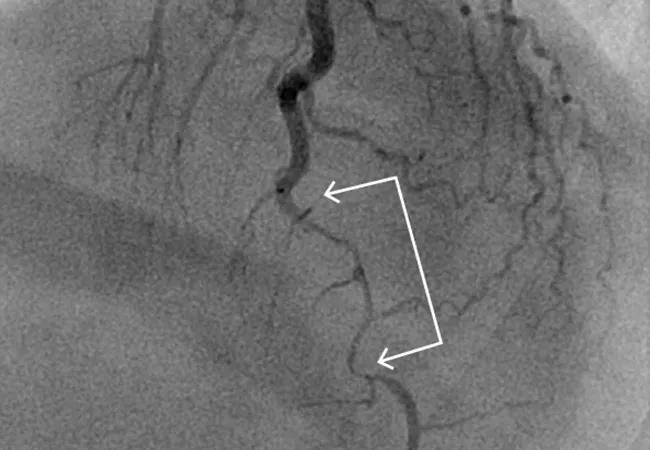
Spontaneous coronary artery dissection (SCAD) was once thought to be a rare condition but is increasingly recognized as a common cause of acute coronary syndrome (ACS), particularly in young women. Despite growing awareness of SCAD — which involves an acute noniatrogenic tear in the coronary artery wall compressing the coronary lumen and possibly causing myocardial infarction — there is a paucity of data on its acute and long-term management.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
To help bridge this gap in care guidance until more evidence is available, a team of Cleveland Clinic experts recently published a review summarizing the literature on treatment of SCAD and describing a comprehensive stepwise management strategy used at Cleveland Clinic. The review, published in the Cleveland Clinic Journal of Medicine (2021;88[11]:623-629) and summarized here, offers guidance on everything from the role of revascularization and medical therapy to long-term follow-up
“Treatment of spontaneous coronary artery dissection has traditionally paralleled medical management of atherosclerotic ACS, but SCAD’s distinct pathophysiology demands that we consider other strategies,” says the paper’s corresponding author, Leslie Cho, MD, Director of the Women’s Cardiovascular Center and Section Head of Preventive Cardiology and Rehabilitation at Cleveland Clinic.
She notes that a growing body of observational data is helping better define SCAD care, but no randomized controlled trials and only a few large prospective studies have focused on SCAD, and few studies have reported outcomes beyond the first few years after dissection. “This has resulted in heterogeneous practice patterns,” Dr. Cho says.
The review notes that the true prevalence of SCAD is unknown, largely due to suspected underdiagnosis. The condition is overwhelmingly seen in women, particularly young women lacking classic cardiovascular risk factors. Just 10% of cases occur in men, often following a physical stressor such as vigorous exercise or heavy lifting. Studies suggest that SCAD accounts for up to 35% of ACS in women under age 60.
Advertisement
A number of conditions have been associated with SCAD, most notably fibromuscular dysplasia, and less commonly chronic systemic inflammatory disease, connective tissue disorders, pregnancy and hypothyroidism.
“Diagnosis of SCAD requires a high index of suspicion in all young patients presenting with ACS, particularly women without traditional risk factors for atherosclerosis,” says co-author Pulkit Chaudhury, MD, a cardiovascular medicine specialist in the Section of Vascular Medicine. He notes that while a variety of imaging studies are helpful in SCAD diagnosis, instrumentation of the vessel wall is associated with risk of propagating the dissection. “For this reason, intracoronary imaging is reserved for clarifying the diagnosis or guiding percutaneous coronary intervention (PCI),” he says.
The paper notes that, like ACS caused by disruption of atherosclerotic plaque, SCAD is most commonly diagnosed during coronary angiography. Unlike in plaque rupture, however, the angiographic appearance of a dissected coronary artery includes multiple radiolucent lines, contrast staining with delayed clearance, and diffuse long narrowing without evidence of significant atherosclerosis in other coronary arteries.
“Coronary angiography is considered the gold standard to confirm SCAD,” Dr. Chaudhury says, “but if it’s not definitive, an adjunctive technique such as intravascular ultrasonography or optical coherence tomography may be used.”
Once the diagnosis of SCAD is established, treatment diverges from that for atherosclerotic ACS. “Although culprit-lesion revascularization is the cornerstone of atherosclerotic ACS management,” Dr. Cho notes, “SCAD is primarily managed medically in most clinically stable patients.” She cites two main reasons:
Advertisement
Observational studies have consistently shown that PCI in the setting of SCAD is associated with worse outcomes and high complication rates, the authors note. Despite these often poor outcomes, revascularization may be appropriate in patients with the following high-risk features:
In SCAD, the goal of PCI is to improve baseline TIMI flow by at least 1 grade or to maintain or achieve TIMI grade 2 or 3, and to reduce residual stenosis to less than 50%.
Antiplatelet therapy. In the absence of randomized controlled trials to guide antiplatelet therapy for SCAD, clinicians have needed to extrapolate data from traditional atherosclerotic ACS literature, the authors note. Aspirin is widely used based on its favorable side-effect profile and well-established benefits in traditional atherosclerotic ACS. Patients who undergo stent placement should be treated in keeping with current ACS guidelines and receive dual antiplatelet therapy consisting of aspirin and a P2Y12 inhibitor for 12 months. For patients who do not undergo PCI, the addition of a second antiplatelet agent is controversial in view of current limited evidence.
Other pharmacologic therapy. The authors’ guidance for other medications for SCAD is as follows:
Advertisement
Chronic management of SCAD is based on the following key principles.
Screen for fibromuscular dysplasia and other arteriopathies.Fibromuscular dysplasia is the condition most commonly associated with SCAD, with an estimated prevalence of around 30% in patients with SCAD. Other arteriopathies associated with SCAD include Marfan syndrome, Loeys-Dietz syndrome, vascular Ehlers-Danlos syndrome, alpha-1 antitrypsin deficiency and polycystic kidney disease.
Given these associations, a vascular medicine evaluation is recommended for all patients diagnosed with SCAD. The evaluation should include a comprehensive vascular history, examination and brain-to-pelvis imaging. Patients with a family history or physical examination findings suggestive of known arteriopathies may benefit from a genetics evaluation. Coronary CT angiography is preferred for imaging when possible, as it has higher spatial resolution than magnetic resonance angiography or ultrasonography. “Screening provides valuable data to guide management, develop a longitudinal follow-up plan and inform prognosis,” says Dr. Chaudhury.
Monitor for chest pain and SCAD recurrence.Although patients with SCAD have a favorable prognosis for long-term survival, they are at risk of chronic angina, noncardiac chest pain and recurrent SCAD. Optimal management requires regular follow-up with a cardiologist experienced in SCAD management.
Of the approximately 20% of patients with SCAD who are readmitted within 30 days of their index event, many develop chronic nitrate-responsive chest pain. This is suspected to be related to coronary microvascular dysfunction, which is common in this population. A long-acting nitrate and/or calcium channel blocker may be considered as needed.
Advertisement
SCAD recurrence rates of approximately 10% to 27% have been reported in the literature. For this reason, the authors recommend close monitoring of patients for new or worsening cardiac symptoms, which should prompt further testing. “While no consensus-based recommendations for a particular noninvasive imaging method have been developed,” Dr. Chaudhury notes, “we find positron emission tomography stress imaging with coronary flow reserve most helpful, as it allows evaluation of ischemia in the previously affected territory as well as microvessel dysfunction.” He adds that coronary CT angiography can be beneficial for follow-up and that cardiac MRI is useful in stable patients with recent SCAD and suspicion for pericarditis.
Recommend cardiac rehabilitation.Cardiac rehabilitation is an important component of management following SCAD, but it remains significantly underused despite clear short- and long-term benefits for perceived physical and mental health and a consistent record of safety in patients with SCAD.
“We recommend referring all patients with SCAD for cardiac rehabilitation, including young women without other comorbidities,” says co-author Erik Van Iterson, PhD, MS, Director of Cardiac Rehabilitation at Cleveland Clinic. “Our aerobic exercise plan is tailored to each patient, typically involving training set at a target heart rate zone equivalent to 50% to 70% heart rate reserve based on initial exercise stress testing.” He adds that the frequency of cardiac rehabilitation and aerobic exercise should be at least three times weekly for 20 to 30 minutes to start. As patient fitness and comfort gradually improve, program progression should be encouraged but implemented conservatively. “The long-term goal is for patients to comfortably exercise 45 to 60 minutes per session on most days of the week,” Dr. Van Iterson explains. Resistance training involving isometric contraction should be avoided in patients with a history of SCAD, and strength training should be limited to light intensity and involve no breath-holding.
The authors conclude their review with the figure below presenting the stepwise approach to management of SCAD used at Cleveland Clinic, reflecting the principles outlined above.
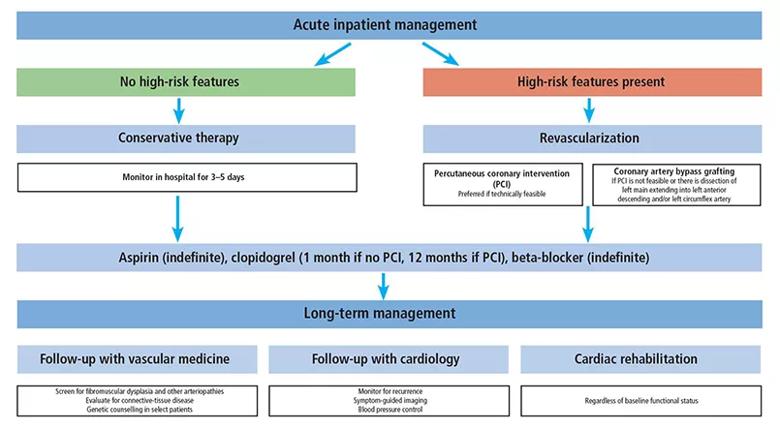
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/80cc1282-54a6-4625-bb63-61079de39403/22-HVI-2713173-CQD-Inset_jpg)
Figure. Schematic showing Cleveland Clinic’s approach to SCAD management.
They also issue a call for prospective, randomized controlled studies to facilitate development of an evidence-based treatment algorithm for SCAD, particularly addressing the role and duration of dual antiplatelet therapy, the use of statin therapy, and indications for and timing of revascularization. “With increasing clinician awareness of SCAD and advancements in angiographic diagnostic techniques, SCAD diagnoses are increasing,” Dr. Cho observes. “This makes the need for a clear treatment approach all the more pressing.”
Advertisement

How Cleveland Clinic is using and testing TMVR systems and approaches

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients

Multimodal evaluations reveal more anatomic details to inform treatment

Insights on ex vivo lung perfusion, dual-organ transplant, cardiac comorbidities and more