Survival for months or several years while delaying treatment is possible
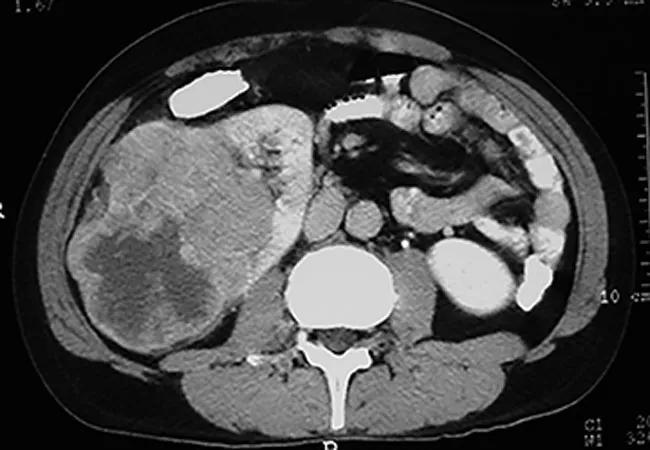
Certain carefully selected metastatic renal cell carcinoma (mRCC) patients who choose close monitoring rather than immediate systemic therapy can live for months or several years before cancer progression while avoiding the significant burdens of treatment, a Cleveland Clinic-led study has found.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Active surveillance, when properly applied, doesn’t appear to compromise eventual systemic therapy, reduce overall survival or worsen patients’ emotional state during the waiting period, the researchers determined.
More research is needed to validate the results and to explore the risks and benefits of surveillance as more novel therapies become available, says the study’s principal investigator, Brian Rini, MD, FACP. But the phase 2 study indicates that delayed treatment with close monitoring is a safe, viable approach for some mRCC patients with limited metastatic disease and limited risk factors, and provides guidance for clinicians applying the strategy.
“There is a public perception that all cancers should be treated immediately because they are equally lethal,” Dr. Rini says. “But what we’ve seen in this small clinical trial is that a subset of adults with advanced kidney cancer have slow-growing disease that can be safely managed using active surveillance. That could spare those patients the inconvenience and debilitating side effects of aggressive treatments for about a year, and in some cases several years, without worsening anxiety and depression.”
mRCC can have a highly variable course. Previous research involving patients who received immediate therapy showed that favorable-risk patients with zero pre-treatment clinical features associated with reduced survival had a median survival time of 20 months, while poor-risk patients with three or more risk factors had a median survival time of four months.
Advertisement
Although systemic treatment employing tyrosine kinase inhibitors of the vascular endothelial growth factor can extend survival in mRCC patients and is the standard of care, this approach is palliative, not curative, and requires chronic therapy, with significant toxicity and morbidity and entailing considerable time commitment and expense for patients.
Active surveillance is a clinical tactic often employed with localized RCC but infrequently in patients with advanced/metastatic disease. Intuitively, active surveillance as an initial strategy would seem to offer clinical and quality-of-life benefits for mRCC patients who show indolent growth of metastases. Several small retrospective reports and a randomized discontinuation trial involving deferred treatment in mRCC patients have suggested that delays do not have an adverse impact. However, prospective assessment of active surveillance in mRCC had not been undertaken prior to this study.
Between 2008 and 2013, Dr. Rini and colleagues at five medical centers in the United States, Spain and the United Kingdom enrolled 52 treatment-naïve mRCC patients. Four were excluded from the analysis, leaving a cohort of 48. Participants’ decisions to take part and thus choose active surveillance over immediate systemic treatment were made jointly with their treating physician, as were decisions to discontinue observation and initiate systemic therapy.
Study participants underwent baseline and regular repeat CT scans and clinical assessments to determine change in tumor burden and time to progression. They also were periodically assessed for quality of life, anxiety and depression status changes. Overall survival, progression-free survival and best overall response after therapy (the latter for those participants who discontinued active surveillance and initiated treatment) were recorded.
Advertisement
Participants were followed for a median of 38.1 months. Forty-three of the 48 patients had confirmed disease progression at some point in the study; 37 discontinued surveillance and began systemic therapy after progression while six continued on surveillance. The median time on surveillance was 14.9 months.
Of the 43 patients who experienced progression while on surveillance, 32 had growth in existing metastases sites, eight had new metastases sites and three had both. In the 42 patients who did not undergo tumor resection while on surveillance, the median change in tumor burden was 1.3 cm, with a median growth rate of 0.09 cm per month.
Twenty-two (46 percent) of the 48 study participants died, all from mRCC. Estimated median overall survival from the start of surveillance was 44.5 months.
Of the 31 patients who discontinued surveillance and for whom subsequent systemic therapy data were available, 10 (32 percent) had objective partial responses. Median overall survival was 38.6 months. Progression-free survival was not assessed.
Patients’ anxiety, depression and quality-of-life questionnaire scores during the course of surveillance did not change significantly compared with baseline, indicating that delaying treatment did not have a negative impact on their emotional status.
The study was a single-arm design, so it is not possible to directly compare outcomes of surveillance patients with those of a concurrent group who received immediate systemic therapy. However, the surveillance patients’ median survival rates and objective response rates to subsequent treatment are indicators that it is a safe, viable approach, Dr. Rini says.
Advertisement
“Median survival in metastatic kidney cancer in recent trials has been about 30 months,” he says. “Our active surveillance patients’ estimated median survival was 44.5 months. Much that is attributable to patient selection — patients had to have slow-growing disease to qualify for the observational approach on the trial. But it didn’t appear that surveillance compromised survival. Similarly, when we looked at response to subsequent therapy, it looked the same or better than in historical controls. It’s not a perfect comparison, but it suggests that these active surveillance patients do just as well when you start them on treatment.”
The researchers’ analysis of baseline clinical characteristics showed that 29 patients with one or no International Metastatic Database Consortium (IMDC) adverse risk factors and two or fewer organs with metastatic disease had an estimated median surveillance time of 22.2 months, while all other patients with more IMDC adverse risk factors and multiple metastases sites had an estimated median surveillance time of 8.4 months
Clinicians can use those clinical characteristics in a prognostic manner to identify patients for whom active surveillance may be a successful strategy. Two patients under surveillance in the study developed new central nervous system (CNS) metastases, highlighting the importance of routine CNS imaging during the observation period.
“Active surveillance is not going to be the appropriate approach for every patient with metastatic renal cell carcinoma,” Dr. Rini says. “From years of seeing the disease, I would estimate that it’s one or two patients out of 10 that could be watched. It wasn’t completely unheard of for a physician to recommend delayed treatment, but it was based on experience and a bit of gut instinct. It had never to our knowledge been studied prospectively. I think that’s the value our trial added — examining the selection criteria and more precisely defining the outcome of the surveillance approach.”
Advertisement
Advertisement

One pediatric urologist’s quest to improve the status quo

First single-port renal vein transposition reduces recovery time and improves outcomes
Fully-automated process uses preop CT, baseline GFR to estimate post-nephrectomy renal function
Belzutifan superior to everolimus in phase 3 clinical trial
Management of high-risk RMSK in the pre-and current eras of neoadjuvant therapy
NIH-funded study explores novel MRI technique to stage cystic kidney disease
AI-generated model bests predictive abilities of human experts
Study highlights benefits of nephrologist-led urine sediment analysis