CT imaging using radiopaque markers can help assess healing after surgery
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/45eac8b4-aa1f-4220-ae88-403cc2fa665b/23-ORI-4143760-CQD-650x450-1_jpg)
23-ORI-4143760 CQD 650×450
By Kathleen A. Derwin, PhD; Eric T. Ricchetti, MD; Joseph P. Iannotti, MD, PhD; and other members of the Cleveland Clinic Shoulder Group
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
More than half of people age 70 and older exhibit rotator cuff disease,1 and at least one in 20 Americans has rotator cuff repair (RCR) surgery by age 65.2 Over 250,000 RCRs are performed annually in the U.S.,3 at an estimated $3 billion in medical costs.4 Reports of ultrasound and MRI-assessed structural outcomes show that 20%-70% of RCRs fail by re-tearing,5-7 typically within six months of surgery.8,9
Paradoxically, patients believed to have an intact repair sometimes demonstrate persistent or even progressive degenerative muscle changes and shoulder weakness.10,11 We postulate that the weak relationships between current measures of structural integrity of the repair and such clinical outcomes are due to an imprecise understanding of tendon healing. This imprecise understanding limits advances in treatment and rehabilitation strategies aimed to improve healing and outcomes.
Therefore, the objective of our research is to challenge and expand the current definition of rotator cuff healing by investigating tendon retraction — broadly defined as translation of the repaired tendon away from the bone with or without a recurrent defect — as a common and clinically predictive structural outcome following RCR.
Currently, rotator cuff healing following RCR is defined on postoperative MRI or ultrasound as “intact,” “attenuated,” “partially re-torn” or “failed” based on signal abnormalities and the observation of a recurrent defect. Yet clinical imaging modalities are inadequate to discern the extent to which the repaired tendon has “failed with continuity” (undergone significant retraction in the absence of a recurrent defect).12
Advertisement
To address the need for more objective and precise measures of tendon healing, we have developed a method using CT imaging of radiopaque markers to monitor tendon retraction in patients.12,13 We have shown that tendon retraction of more than 5 mm can be considered meaningful above the usual range of measurement variation.14
Measurements of tendon retraction allow us to investigate the clinical implications of translation of the repaired tendon away from the bone with or without a recurrent defect. We hypothesize that “failure with continuity” is a common yet unrecognized structural phenomenon of rotator cuff healing that is significantly and meaningfully correlated with clinical outcomes.
To investigate tendon retraction after RCR and its relationship with postoperative repair integrity and shoulder function,we enrolled 117 patients undergoing primary, arthroscopic double-row (or double-row equivalent) repair of the supraspinatus and/or infraspinatus tendons into a prospective cohort study. All surgeries were performed by one of seven Cleveland Clinic shoulder surgeons between 2016 and 2021.
At the time of RCR, one to four radiopaque markers were tied to the superficial surface of the repaired tendon(s) just medial to the repair site. Each patient had shoulder imaging preoperatively (MRI only), on the day of surgery (CT only), and three, six, 12 and 24 months after surgery (both). Tendon retraction was quantified at each postoperative time by measuring the change in distance from the radiopaque markers to the humeral greater tuberosity on CT images12,13,15 and taking the mean over each patient’s markers (Figure 1).
Advertisement
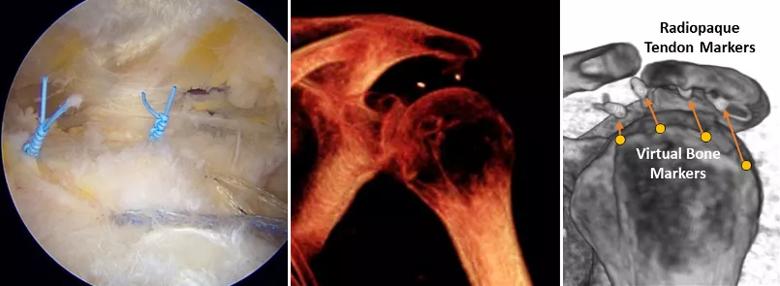
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/dbbe15d6-d0e9-4353-b590-7a24b8c502a9/23-ORI-4143760-CQD-INSET-1_jpg)
Figure 1. Intraoperatively, radiopaque tissue markers were tied to the superficial surface of the repaired tendon just medial to the repair site (left). Each patient had a low-dose CT imaging study on the day of surgery (middle) to establish the baseline distance between the markers and the humeral greater tuberosity. After volumetric registration of the humerus to its position on day of surgery, tendon retraction was quantified at each postoperative time by measuring the change in distance from the radiopaque markers to virtual markers on the humeral greater tuberosity (right) and taking the mean over each patient’s markers. The CT image on the right originally appeared in JSES International and is licensed by Creative Commons CC-BY-NC-ND.14
Two musculoskeletal radiologists blinded to the tendon retraction and clinical data assigned a Sugaya score of repair integrity to the MRI studies.16 Preoperatively and at each postoperative assessment, patients also completed patient reported outcome measures (PROMs) (Penn Shoulder Score, PSS) and were tested for isometric abduction strength (only postoperatively) and active range of motion (ROM) in the scapular plane.
Baseline demographics, tear characteristics and clinical assessment of the cohort are shown in Table 1. Of the 117 original patients, 113 completed two-year follow-up, at which time 19 (17%) had a full-thickness re-tear (Sugaya 4/5). Mean PSS (93±10.9 points at two years), abduction strength (8±4.1 lbf at six months; 12±5.3 lbf at two years) and active abduction ROM (155±18 degrees at two years) improved across the cohort postoperatively.
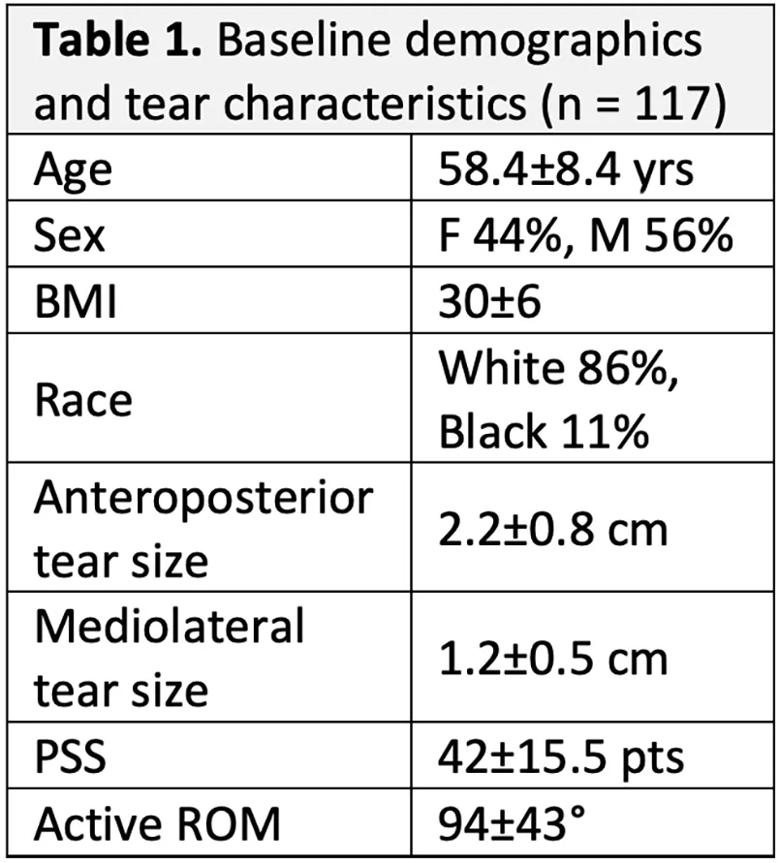
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/5c906dab-8d5a-4717-bb6b-b234da50b398/23-ORI-4143760-CQD-Table-1_jpg)
Tendon retraction increased in all patients after surgery, averaging 13.4±7.9 mm at two years (range, 4.4-46.4 mm), with most retraction occurring by six months (Figure 2). Patients with a Sugaya 4/5 full-thickness re-tear averaged appreciably more tendon retraction at all postoperative times than those with an intact repair or partial-thickness re-tear (Sugaya 1/2/3) (Figures 2 and 3). However, 53% of patients with an intact repair or partial-thickness re-tear had mean tendon retraction greater than 10 mm at two years (Figure 3).
There was considerable overlap in PSS, abduction strength and ROM outcomes between patients with and without a full-thickness re-tear at two years (Figure 3).
Advertisement
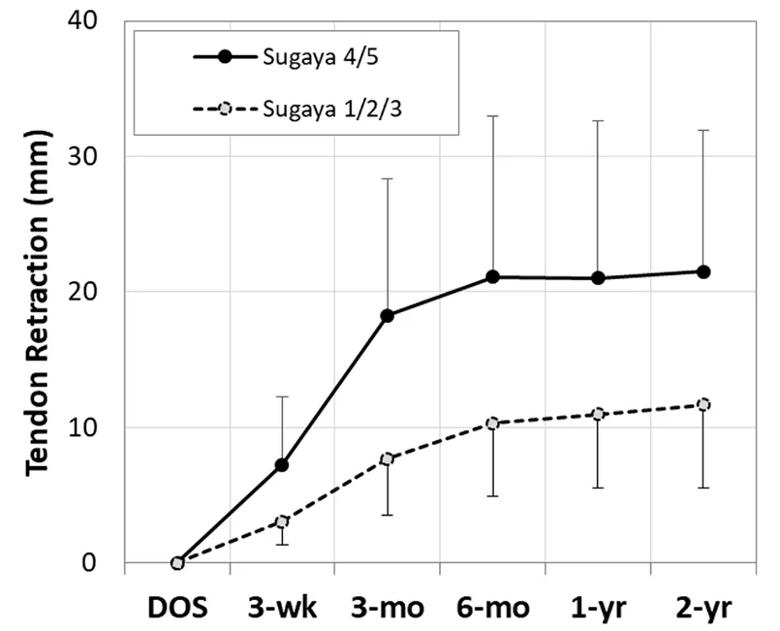
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/35d66fab-f7b7-49f7-a81b-6ff3d6e19392/23-ORI-4143760-CQD-INSET-2_jpg)
Figure 2. Mean tendon retraction in patients with Sugaya score 1/2/3 vs. 4/5 during two years after RCR.
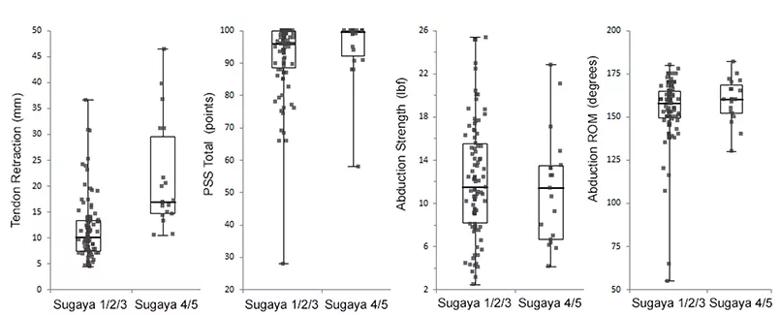
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/867b54dd-90bf-49d9-bef9-60a9529d3828/23-ORI-4143760-CQD-INSET-3_jpg)
Figure 3. Individual patient tendon retraction, PSS total, shoulder abduction strength, and range of motion at two years (n = 113) grouped according to Sugaya score (1/2/3: 83%; 4/5: 17%).
Half of RCR patients with an intact repair or partial-thickness re-tear at two years had significant tendon retraction greater than 10 mm, suggesting that these patients have undergone some degree of “failure with continuity.”12 At two years, patients with a full-thickness re-tear had overlapping distributions of PSS, shoulder abduction strength and ROM with patients with an intact repair or partial-thickness re-tear, but greater tendon retraction, suggesting that tendon retraction may provide more sensitive information about repair integrity and postoperative shoulder function than standard clinical imaging assessments.
Multivariable analysis to investigate relationships between RCR healing (by tendon retraction and/or Sugaya score) and PROMs, abduction strength and/or ROM is ongoing. We anticipate that incorporating postoperative tendon retraction as well as the integrity of the repaired tissue from MRI into the assessment of structural outcomes following RCR will yield an improved understanding of rotator cuff tendon healing as well as a novel quantitative metric to evaluate treatments aimed to improve healing.
Dr. Derwin is staff at Lerner Research Institute, Vice Chair of Biomedical Engineering and co-Director of the Musculoskeletal Research Center at Cleveland Clinic. Dr. Ricchetti is an orthopaedic surgeon and Center Director for Shoulder Surgery in the Department of Orthopaedic Surgery. Dr. Iannotti is an orthopaedic surgeon and Chief of Staff of Cleveland Clinic Florida. Other members of the Cleveland Clinic Shoulder Group include Mark Schickendantz, MD; Lutul Farrow, MD; Alfred Serna, MD; Vahid Entezari, MD; Jason Ho, MD; Carl Winalski, MD; Joshua Polster, MD; Sambit Sahoo, MD, PhD; Bong-Jae Jun, PhD; and Peter Imrey, PhD.
Advertisement
This work was funded by a grant from the National Institutes of Health (5R01AR0608342). Drs. Derwin, Iannotti and Sahoo are inventors with rights to royalties for the radiopaque tissue marker licensed to Viscus Biologics and used in this research.
Advertisement
How it’s similar but different from the direct anterior approach
Collaboration must cross borders and disciplines
Systematic review of MOON cohorts demonstrates a need for sex-specific rehab protocols
Should surgeons forgo posterior and lateral approaches?
How chiropractors can reduce unnecessary imaging, lower costs and ease the burden on primary care clinicians
Why shifting away from delayed repairs in high-risk athletes could prevent long-term instability and improve outcomes
Multidisciplinary care can make arthroplasty a safe option even for patients with low ejection fraction
Percutaneous stabilization can increase mobility without disrupting cancer treatment