What is female hypoactive sexual desire disorder and how is it treated?
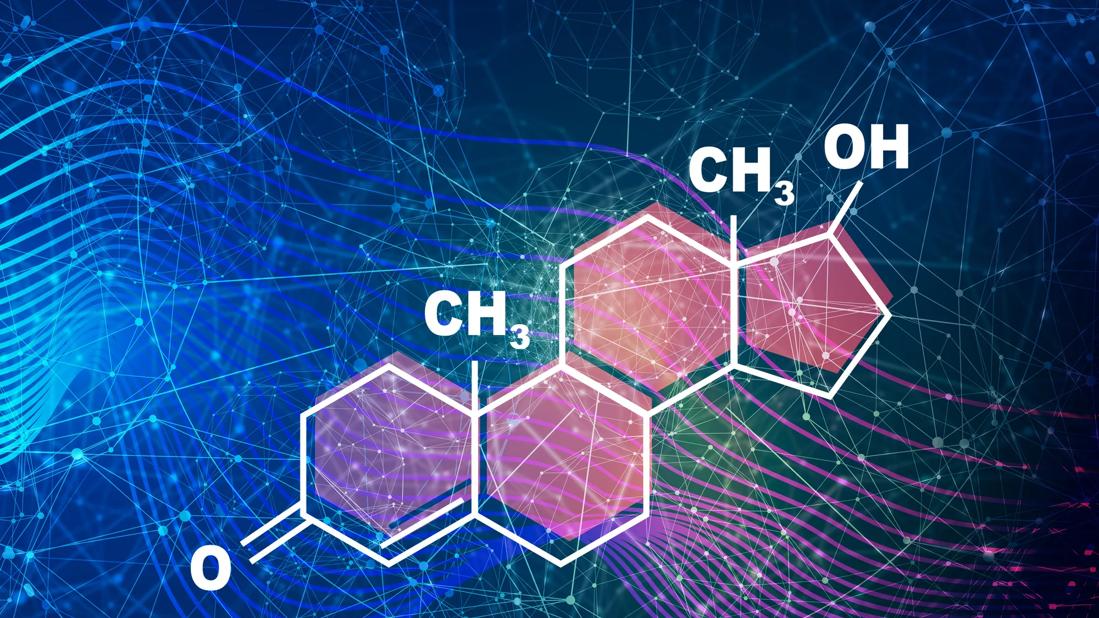
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/4ef35ec4-aff3-4eb9-9c5c-f884e68162d4/testosterone-1386603024)
Testosterone
By Taryn Smith, MD, NCMP, and Pelin Batur, MD, FACP, NCMP
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Please note: This is an abridged version of an article recently published in the Cleveland Clinic Journal of Medicine. To read it in its entirety, including a complete list of references, please visit: https://www.ccjm.org/content/88/1/35
Estrogens are the principal sex hormones responsible for female reproductive maturation and sexual characteristics. However, androgens are also important for female sexual health and well-being.1 The physiologic effects of androgens are in part due to their role as precursors for estrogen synthesis, but these hormones also have independent effects on female reproductive tissues, mood, cognition, breasts, bones, muscles, vasculature and other systems.1
The only evidence-based indication for testosterone therapy in women is to treat hypoactive sexual desire disorder after menopause.7,28,29 Several randomized controlled trials have established the short-term safety and efficacy of prescribed testosterone in women when doses approximate physiologic levels. Currently, no testosterone formulation for women has been approved by the US Food and Drug Administration (FDA), though vaginal DHEA (prasterone) has been approved for treatment of moderate to severe dyspareunia.
Female sexual dysfunction is multifactorial, often influenced by biologic, emotional, cultural and interpersonal factors. It can manifest as decreased sexual desire, painful intercourse, and diminished arousal or orgasmic response, or both.
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)30 merged the previous diagnoses of female hypoactive sexual desire disorder (HSDD) and female arousal disorder (FAD) into a single diagnosis of female sexual interest/arousal disorder (FSIAD). While HSDD and FAD have overlapping features, the Fourth International Consultation on Sexual Medicine and the International Society of the Study of Women’s Sexual Health (ISS-WSH) recommend against combining these diagnoses for clinical purposes.31,32 According to the ISSWSH, HSDD can be defined by 6 or more months of any of the following32:
Advertisement
Randomized controlled trials in postmenopausal women who reported decreased sexual desire have demonstrated that testosterone therapy positively influences sexual function, though none have defined target serum androgen levels that correlate with sexual function.8,27
Four 24-week randomized controlled trials in natural and surgically menopausal women with sexual dysfunction compared transdermal testosterone patches (300 μg/day) and placebo.33-36 The patch significantly increased sexual desire and sexually satisfying events while decreasing sexual-related distress (in three of four studies) compared with placebo. In one study,36 sexually satisfying events increased by 2.1 episodes per month with the transdermal patch compared with 0.98 with placebo (P < .001).
The safety and efficacy of the patch in postmenopausal women was also evaluated in the 52-week APHRODITE study (A Phase III Research Study of Female Sexual Dysfunction in Women on Testosterone Patch Without Estrogen).37 Two doses, 150 μg/day and 300 μg/day, were compared with a placebo. Significant improvements were noted in sexual desire and sex-related distress in both treatment groups (300 μg/day, P < .001, and 150 μg/day, P = .04). Adverse events were minimal, the most common being application-site reaction, acne, breast pain, headache and hirsutism. All metabolic markers remained stable, including liver enzymes, serum lipids, complete blood cell counts and metabolic panels.
Many randomized controlled trials have shown insignificant changes in levels of total or free testosterone after treatment with the 300 μg/day transdermal testosterone patch.27
Advertisement
A transdermal testosterone spray was studied in a randomized controlled trial in women with FSIAD and low serum free testosterone. The self-reported frequency of satisfactory sexual events was greater with active treatment than with a placebo.38
A 12-week, double-blind randomized controlled trial found improved well-being (P = .003), mood (P < .06) and sexual function (P < .001) in women receiving testosterone cream 10 mg/day compared with placebo.39 During the study period, serum testosterone levels stayed in the high-normal range for postmenopausal women, and no adverse events were noted.
In contrast, two large phase 3 randomized controlled trials investigating transdermal testosterone gel 0.22 g/day in women with sexual dysfunction showed no statistically significant differences in sexually satisfying events or sexual desire between treatment and placebo groups.28 Inconsistencies between studies could be due to dosing and route of administration.
There is sufficient high-quality evidence to support letting postmenopausal women with HSDD try testosterone, for a short time, after other reasons for their sexual concerns have been excluded. Evidence is lacking to support the use of testosterone for sexual dysfunction in premenopausal women.
Before starting testosterone therapy for HSDD, all women should undergo a thorough medical and psychosocial assessment and physical examination. Other contributors to symptoms should be identified and addressed before starting testosterone therapy, including medication side effects, mood disorders, relationship concerns, or the genitourinary syndrome of menopause.
Advertisement
Though there is evidence of benefit, currently there are no approved testosterone formulations for women in the United States. Regulated, safe delivery methods are needed.
Systemic DHEA therapy has not been shown to improve symptoms of sexual dysfunction in women who have normal adrenal function.7
For a complete list of references, please visit: https://www.ccjm.org/content/88/1/35
Advertisement
Advertisement
A Q&A with Cleveland Clinic’s board-certified pediatric and adolescent gynecologist
Increasing uptake remains a challenge
Multidisciplinary teams work together in in-situ scenarios
Uterine transposition cleared the field for radiation therapy
ACOG-informed guidance considers mothers and babies
Prolapse surgery need not automatically mean hysterectomy
Artesunate ointment shows promise as a non-surgical alternative
New guidelines update recommendations