First in-human-trial for diagnostic bladder disorder device
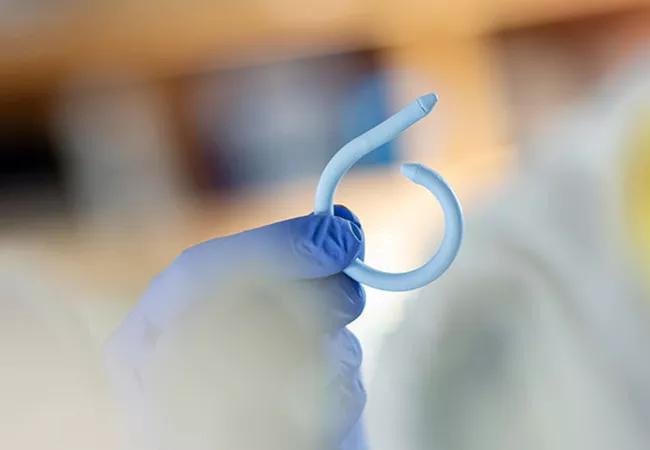
The UroMonitor, a wireless, insertable pressure sensor to assist in the diagnosis of urinary incontinence and other bladder disorders is safe, feasible and well-tolerated in women with refractory overactive bladder (OAB), according to the interim results of a Cleveland Clinic-led proof-of-concept study.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The data were presented at the American Urological Association 2021 Annual Meeting. It also received the Best Paper Award from the Engineering and Urology Society.
Margot Damaser, PhD, and colleagues in the Biomedical Engineering Department of Cleveland Clinic’s Lerner Research Institute, have been developing the device for more than a decade. But up until now, its clinical utility has been unknown.
Dr. Damaser, who has referred to the device as a “Fitbit for the bladder,” and the team of clinical collaborators, including Howard Goldman, MD, urologist and one of the co-authors of the study, say the results so far are promising.
Eleven adult female patients, all of whom were evaluated for refractory OAB, were included in this study. Investigators first performed a baseline assessment using standard multi-channel urodynamics (USD).
Next, they inserted the device transurethrally, as would be done in cystoscopy or catheter placement, with a silk suture attached to one end of the device and taped to the patients’ thighs for easy retrieval from the bladder.
Its medical silicone-coated sheath that houses the pressure-sensing technology curls into a pigtail shape upon insertion to remain within the bladder, explains Dr. Goldman. The device transmits vesical pressure data at 10 Hz to a device taped to the patients’ abdomens.
Once the USD catheters were removed, leaving only the UroMonitor in place, the patients were encouraged to ambulate and void. The team assessed patient discomfort at every stage using visual-analog pain scales, and overall comfort and safety during testing were also assessed.
Advertisement
The researchers have reported data from three of 11 patients so far, but Dr. Goldman notes they now have data from all of the patients, which will soon publish.
“The device is easy to insert and extract; it reliably reproduced vesical pressure data patterns during both filling cystometry and ambulatory measurements. And patients were able to void freely with the device in place,” he says.
Patients reported minimal discomfort during the insertion phase, but nothing unexpected, says Dr. Goldman. There were no post-procedure complications to report and, anecdotally, he says, physicians and patients are pleased with the experience.
Dr. Goldman says that in its first-in-human trial, the UroMonitor demonstrates its utility as a data collection tool that does not hinder normal activity and can identify the same bladder events as that of a conventional UDS.
The team has already begun another arm of the trial to test the device in patients with multiple sclerosis. The researchers are hopeful that the device may one day play a therapeutic role for patients with neurogenic etiologies, noting that possible applications include controlled drug delivery and feedback to neuromodulation or electrical stimulation systems. The device may also restore sensation for patients with pelvic floor dysfunction or inform them about when to empty their bladder.
For now, though, they are pleased that these preliminary findings show that the device is a safe and effective tool in the evaluation of OAB.
Advertisement
Advertisement

Pediatric urologists lead quality improvement initiative, author systemwide guideline

Fixed-dose single-pill combinations and future therapies

Reproductive urologists publish a contemporary review to guide practice

Two recent cases show favorable pain and cosmesis outcomes

Meta-analysis assesses outcomes in adolescent age vs. mid-adulthood

Proteinuria reduction remains the most important treatment target.

IgA nephropathy is a relatively common autoimmune glomerular disease that can be diagnosed only by biopsy

Oncologic and functional outcomes are promising, but selection is key