Pathologic wear patterns, progression of pathology, rotator cuff fatty infiltration examined
By Eric T. Ricchetti, MD, and Joseph P. Iannotti, MD, PhD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
We conducted three studies to improve our understanding of pathologic wear patterns in glenohumeral osteoarthritis, including factors associated with certain pathologies. Our results:
The Walch classification is the most commonly used method for defining pathology in glenohumeral osteoarthritis and impacts decision-making and outcomes in shoulder arthroplasty. Glenoids are classified (subtypes A1, A2, B1, B2, C) on the basis of the pattern of glenoid morphology and bone loss, and the presence of subluxation of the humeral head. However, there are pathologic patterns not easily classified within the original Walch system, particularly cases with more severe pathology.
We hypothesized that these patterns have important distinguishable anatomic characteristics that may have different implications for clinical outcome and recommended treatment.
The purpose of the study was to use three-dimensional computed tomography (3-D CT) image analysis to define additional pathologic subtypes that can be differentiated from the current Walch classification. We performed quantitative measurements of premorbid and pathologic anatomy using preoperative 3-D CT scans from 155 cases of advanced glenohumeral osteoarthritis that underwent anatomic or reverse total shoulder arthroplasty. We defined premorbid glenohumeral anatomy on the basis of previously validated methods using 3-D glenoid vault and humeral best-fit circle models including the premorbid glenoid version, joint-line medialization, and humeral-glenoid alignment (Figure 1).
Advertisement
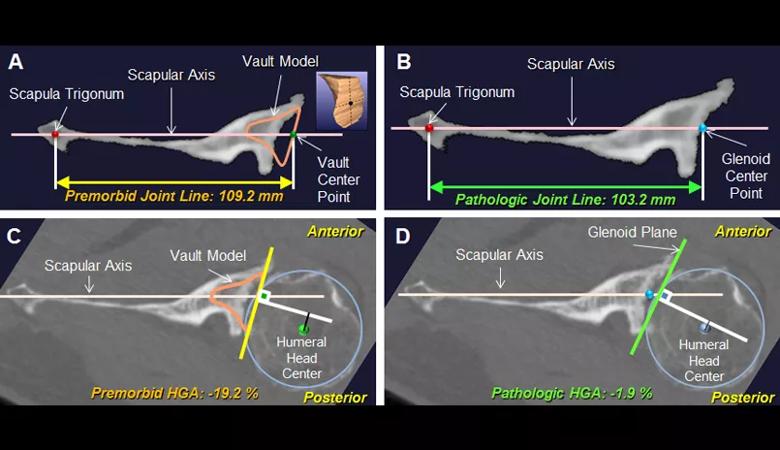
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/75a7f65b-1762-4c47-a5d5-7b9ce20f8ef5/18-ORT-1088-Iannotti-Fig-01-805pxl-width_jpg)
Figure 1: Case example of a B3 glenoid. The premorbid (A) and pathologic (B) joint line and premorbid (C) and pathologic (D) humeral-glenoid alignment (HGA) were measured on the 2-D orthogonal axial image that passed through the glenoid center point. The position of the premorbid joint line was measured as the distance between the vault center point and the scapula trigonum (A). The position of the pathologic joint line was measured as the distance between the glenoid center point and the scapula trigonum (B). Premorbid HGA was measured as the distance (black line) from the humeral head center to the perpendicular line (white) drawn from the vault center point (C). Pathologic HGA was measured as the distance (black line) from the humeral head center to the perpendicular line (white) drawn from the glenoid center point (D).
We determined the anatomic features that differentiate new glenoid morphologic patterns from the existing Walch classification both qualitatively and quantitatively, and defined two new glenoid subtypes (B3 and C2) (Table 1). The B3 glenoid (Figure 2) has high retroversion, normal premorbid version and acquired central and posterior bone loss that, on average, is greater than that of the B2 glenoid.
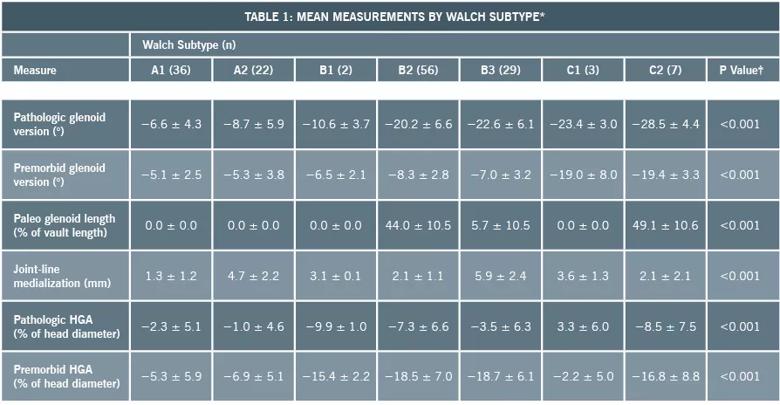
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6c550ff5-0da9-48a8-90d6-0e6f91dca1c3/18-ORT-1088-Iannotti-Table-01-805pxl-width_jpg)
Table 1. *Values are given as the mean and the standard deviation. †For a given measure, the P value represents a significant difference in the mean values across all of the glenoid subtypes in the modified classification system. Post-hoc tests (with Bonferroni multiple-comparison corrections) were then conducted to identify specific glenoid subtypes showing differences on pairwise comparisons.
Advertisement
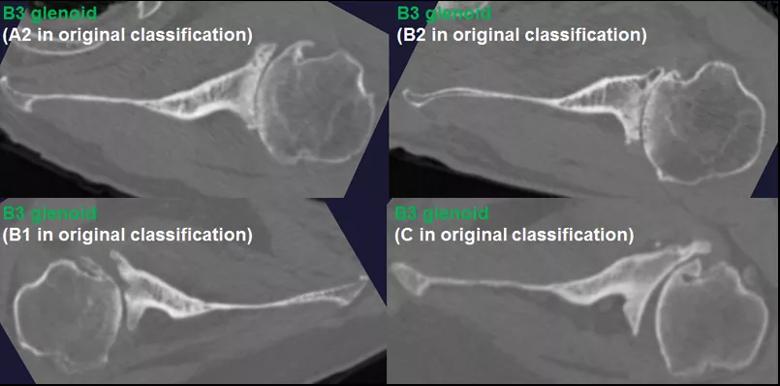
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/fa6cbb32-71df-49d8-9dce-dc3fb09d51e1/18-ORT-1088-Iannotti-Fig-02-805pxl-width_jpg)
Figure 2: The B3 glenoid has both central and asymmetric posterior bone loss, making the joint line more medialized. Unlike the B2 glenoid, the B3 glenoid does not have a paleo glenoid or it is so small that it is difficult to define when present, due to bone loss. Shown are 4 examples of B3 glenoids, with the classification according to the original Walch classification system also noted. The C2 glenoid (Figure 3) is dysplastic with high retroversion, high premorbid version and acquired posterior bone loss, giving it the appearance of a biconcave glenoid with posterior translation of the humeral head. This C2 glenoid can be confused with the B2 glenoid.
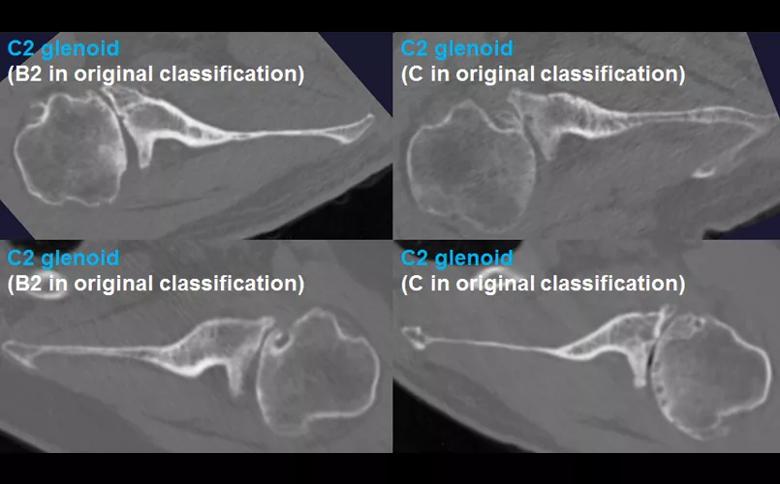
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/ba18ecad-bb9f-4138-a8c7-593c90b19399/18-ORT-1088-Iannotti-Fig-03-805pxl-width_jpg)
Figure 3: The C2 glenoid is similar to the B2 glenoid in that there is a biconcave surface with associated posterior humeral head subluxation; however, the pathologic glenoid retroversion and the premorbid glenoid version are both greater in the C2 compared with the B2 glenoid. Shown are 4 examples of C2 glenoids, with the classification according to the original Walch classification system also noted.
The new B3 and C2 glenoid subtypes may result in different clinical outcomes than classic B2 or C types; therefore, our findings suggest that they should be included in a modified classification system.
While the addition of new Walch subtypes allows for better distinction of more severe pathologic patterns, the progression of glenohumeral osteoarthritis over time and the factors that contribute to progression are still not well defined. We performed a follow-up study with 3-D CT image analysis to determine whether there are common patterns of pathologic progression based on Walch classification and if glenoid bone-loss patterns correlate with rotator cuff fatty infiltration.
Advertisement
Sixty-five cases of glenohumeral osteoarthritis (42 A-type glenoids, 23 B-type glenoids) with at least two shoulder CT scans performed at least 24 months apart were identified. The amount and location of glenoid bone loss was again measured (Figure 1), and rotator cuff fatty infiltration was calculated as a percentage of cross-sectional muscle area.
At an average of 74 ± 32 months after the initial CT scans, only eight out of 42 A1 glenoids had evidence of pathologic progression (five to A2 type, three to B type), whereas 17 of 19 B1 glenoids had progressed (15 to B2, two to B3). This difference was significant on univariate and multivariate analysis (P < 0.001) (Figure 4). The odds of joint line medialization occurring were 8.1 times higher (95% confidence interval [CI]: 2.1-31.4) for B-type glenoids than for A-type glenoids. Among the glenoids that underwent medialization, B-type glenoids showed more medialization over time (estimated change, 0.70 mm/year; P = 0.036), whereas no significant relationship between medialization and time was observed for A-type glenoids (estimated change, 0.013 mm/year; P = 0.95) (Figure 5).
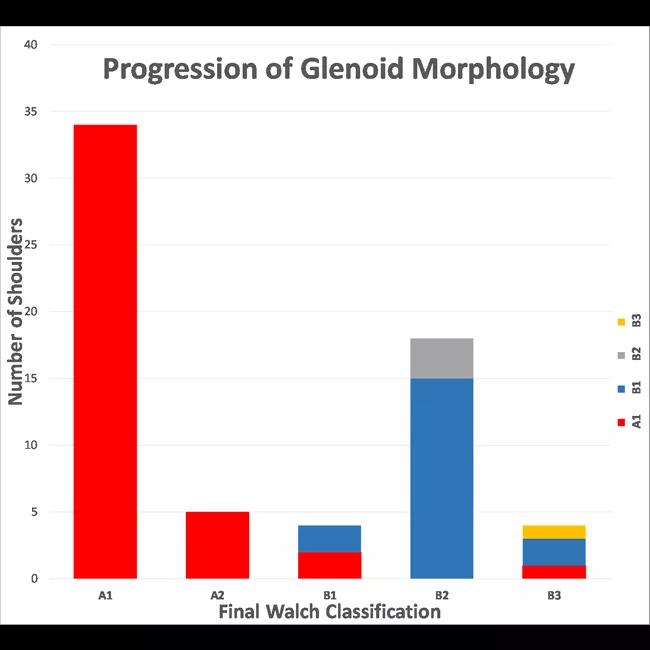
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/80cf7a6a-8691-4af7-aca4-0b38c7245537/18-ORT-1088-Iannotti-Inset-Image-04-650pxl-width_jpg)
Figure 4: Bar graph representing the progression of glenoid morphology according to the modified Walch type. The height of each column represents the total number of shoulders of each Walch type on the last available CT scan, whereas the color(s) of each bar illustrate the initial Walch classification(s) according to the legend along the right side of the figure.
Advertisement
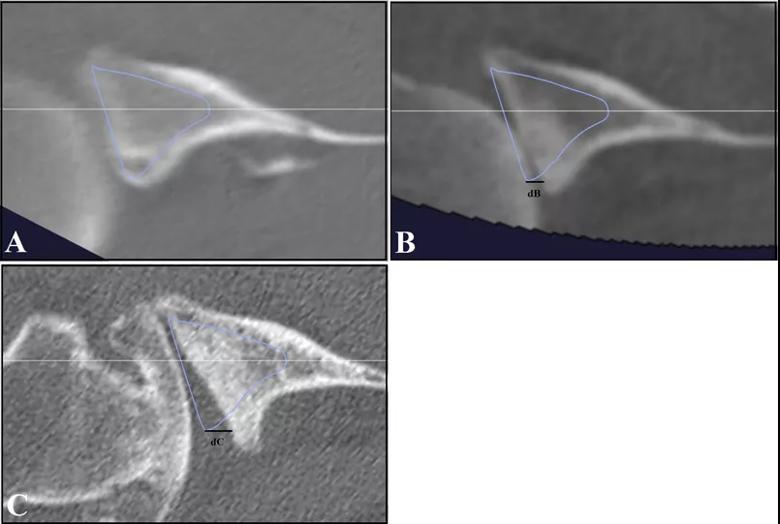
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/96de9122-0583-4a37-8656-5430236fff79/18-ORT-1088-Iannotti-Fig-05-805pxl-width_jpg)
Figure 5: Images of B-type glenoid progression. A B1 glenoid with subluxation of the humeral head is seen at initial CT scan (A), with progression to a B2 glenoid on a 4-year interim CT scan (B), and further progression of posterior glenoid bone loss seen at 10 years (C). The vault model (blue) and the glenoid center axis (white) are depicted. In B-type glenoids, the joint line medialization was measured in reference to the vault model at the posterior aspect of the glenoid. On the initial CT scan, there is no measurable difference between the vault model and the glenoid surface. Line dB (4.22 mm) and Line dC (7.96 mm) show the joint line medialization measurements at 4 years and 10 years, respectively.
The median percent fatty infiltration in the infraspinatus muscle was higher in association with B-type glenoids than in association with A-type glenoids on both initial (14% versus 7%; P < 0.001) and final follow-up (16% versus 10%; P = 0.003) CT scans.
These findings demonstrate that asymmetric bone loss rarely develops in A1 glenoids, whereas initial posterior translation of the humeral head (B1 glenoids) may be associated with subsequent development and progression of posterior glenoid bone loss over time. While differences in fatty infiltration of the posterior rotator cuff were seen between A-type and B-type glenoids, the clinical relevance of this finding is currently unknown.
The association of rotator cuff fatty infiltration and preoperative glenoid morphology as defined by the modified Walch classification was investigated further in another follow-up study. We performed preoperative 3-D CT image analysis in 190 cases of advanced glenohumeral osteoarthritis that underwent anatomic or reverse total shoulder arthroplasty (Figure 1). Rotator cuff fatty infiltration was assessed by the Goutallier classification on the sagittal CT slice just medial to the spinoglenoid notch for each muscle.
There was a significant difference in Goutallier score for the supraspinatus, infraspinatus and teres minor muscles between Walch subtypes (A1, A2, B1, B2, B3, C1, C2) (P ≤ 0.05). High-grade posterior rotator cuff fatty infiltration was present in 55 percent (21 out of 38 cases) of B3 glenoids compared with 8% (three of 39) of A1 glenoids (Table 2). Increasing joint-line medialization was associated with increasing fatty infiltration of all rotator cuff muscles (P ≤ 0.05). Higher fatty infiltration of the infraspinatus, teres minor and combined posterior rotator cuff muscles was associated with increasing glenoid retroversion (P ≤ 0.05). After controlling for joint-line medialization and retroversion, B3 glenoids were more likely to have fatty infiltration of the supraspinatus and infraspinatus muscles than B2 glenoids.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b6cd6e3d-e824-45d0-b15f-7390a9acf595/18-ORT-1088-Iannotti-Table-02-805pxl-width_jpg)
Table 2. *Differences in the distribution of low- and high-grade FI of each rotator cuff muscle among the modified Walch types were assessed. No FI was classified as a Goutallier score of 0 or 1. Low- and high-grade FI included Goutallier scores of ≥ 2. High-grade posterior cuff FI (the sum of the scores for the infraspinatus and teres minor) was a Goutallier score of ≥ 4, and low-grade posterior cuff FI was a score of < 4. A P value of ≤ 0.05 indicates significance.
Findings from this study further define the association of rotator cuff muscle changes to glenoid wear in glenohumeral osteoarthritis, but future studies are needed to determine the causal relationship between these changes and how this impacts pathologic progression over time.
The results of these three studies further define the common bony wear patterns in glenohumeral arthritis, the progression of wear over time and its association with rotator cuff muscle fatty infiltration. These new findings may ultimately impact treatment decision-making with regard to the timing of surgical intervention and the type of shoulder arthroplasty (anatomic versus reverse replacement) that is pursued in a patient with end-stage glenohumeral osteoarthritis that fails nonoperative management in order to maximize clinical outcome and implant longevity.
Dr. Ricchetti is Center Director for Shoulder Surgery in the Department of Orthopaedic Surgery. Dr. Iannotti is Acting Chairman, Department of Orthopaedic Surgery, and Chairman, Orthopaedic & Rheumatologic Institute.
References:
Iannotti JP, Jun BJ, Patterson TE, Ricchetti ET. Quantitative measurement of bony pathology in advanced glenohumeral osteoarthritis. J Bone Joint Surg Am. 2017;99(17):1460-1468.
Walker KE, Simcock XC, Jun BJ, Iannotti JP, Ricchetti ET. Progression of glenoid morphology in glenohumeral osteoarthritis. J Bone Joint Surg Am. 2018;100(1):49-56.
Donohue KW, Ricchetti ET, Ho JC, Iannotti JP. The association between rotator cuff muscle fatty infiltration and glenoid morphology in glenohumeral osteoarthritis. J Bone Joint Surg Am. 2018;100(5):381-387.
Advertisement

How it’s similar but different from the direct anterior approach

Collaboration must cross borders and disciplines

Systematic review of MOON cohorts demonstrates a need for sex-specific rehab protocols

Should surgeons forgo posterior and lateral approaches?

How chiropractors can reduce unnecessary imaging, lower costs and ease the burden on primary care clinicians

Why shifting away from delayed repairs in high-risk athletes could prevent long-term instability and improve outcomes

Multidisciplinary care can make arthroplasty a safe option even for patients with low ejection fraction

Percutaneous stabilization can increase mobility without disrupting cancer treatment