TRISCEND II trial reports 1-year results
Patients with severe or worse tricuspid regurgitation (TR) had better clinical and quality-of-life outcomes one year after transcatheter tricuspid valve replacement compared with patients treated with medical therapy alone. In more than 95% of patients who had valve replacement, TR level improved to mild or better. The same result was seen in 2.3% of patients receiving only medical therapy.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
These one-year results of the multicenter TRISCEND II trial were presented at the 2024 Transcatheter Cardiovascular Therapeutics (TCT) conference and recently published in The New England Journal of Medicine. They supplement the favorable six-month results from the trial’s first 150 patients, presented at the 2023 TCT conference. Those early results supported FDA approval of the Evoque transcatheter tricuspid valve replacement system (Edwards Lifesciences) as a breakthrough device in 2024.
“The novel Evoque system was approved based on limited initial data,” says Samir Kapadia, MD, Chair of Cardiovascular Medicine at Cleveland Clinic and an investigator in the TRISCEND II trial. “Our new one-year data provide more robust evidence that this method of valve replacement is a safe and effective option for treating severe TR, a condition for which we’ve long needed more-effective alternatives to medical therapy and less-invasive alternatives to open valve surgery.”
TRISCEND II researchers at medical centers in the U.S. and Germany prospectively studied 400 patients with TR rated severe (3 on a 0-5 scale, where 0 is none and 5 is torrential) or worse. Patients were randomly assigned to two study groups: 267 to have transcatheter valve replacement with the Evoque system along with medical therapy and 133 to receive medical therapy only (e.g., oral diuretic medication). Patients had a mean age of 79 and either had symptoms of TR or had been hospitalized for heart failure. Most patients were women (75.5%) and had atrial fibrillation (94.9%).
Advertisement
Patients were evaluated in more than 34,000 pairs, with one patient from each study group. The performance of each patient was compared to determine “wins” in various outcome categories. After one year, the valve-replacement patients had more wins than the medical-therapy patients in these outcomes:
However, when compared to the medical-therapy patients, the valve-replacement patients had fewer wins in annualized rate of hospitalization for heart failure (9.7% vs. 10.0%).
The primary outcome was a composite of all of these scores: a win ratio of 2.02 for valve replacement (95% CI, 1.56 to 2.62; P < .001).
“Overall, patients who were treated with transcatheter valve replacement saw better improvement in symptoms and quality of life,” Dr. Kapadia says. “This is promising news for people with severe tricuspid regurgitation, who can experience debilitating fatigue, dyspnea and other symptoms, and have a higher risk of death. We may be able to offer them new hope when medication hasn’t provided relief and when open surgery to replace the valve is too risky.”
Adds Amar Krishnaswamy, MD, Section Head of Invasive and Interventional Cardiology at Cleveland Clinic, “Patients with severe tricuspid regurgitation are often poor candidates for cardiac surgery, even when they are physically active. As such, the ability to replace the valve with a catheter-based system, with the patient often going home either the same day or the next day, is a tremendous step forward.”
Advertisement
In addition to improved clinical and quality-of-life outcomes, the valve-replacement group also recorded a significant reduction in regurgitation compared with the medical-therapy group. Echocardiography-based TR ratings at one year were as follows:
| Severity of tricuspid regurgitation | Valve-replacement patients | Medical-therapy patients |
|---|---|---|
| 0. None | 72.6% | 0.0% |
| 1. Mild | 22.6% | 2.3% |
| 2. Moderate | 3.8% | 13.8% |
| 3. Severe | 0.9% | 41.4% |
| 4. Massive | 0.0% | 19.5% |
| 5. Torrential | 0.0% | 23.0% |
| Severity of tricuspid regurgitation | ||
| 0. None | ||
| Valve-replacement patients | ||
| 72.6% | ||
| Medical-therapy patients | ||
| 0.0% | ||
| 1. Mild | ||
| Valve-replacement patients | ||
| 22.6% | ||
| Medical-therapy patients | ||
| 2.3% | ||
| 2. Moderate | ||
| Valve-replacement patients | ||
| 3.8% | ||
| Medical-therapy patients | ||
| 13.8% | ||
| 3. Severe | ||
| Valve-replacement patients | ||
| 0.9% | ||
| Medical-therapy patients | ||
| 41.4% | ||
| 4. Massive | ||
| Valve-replacement patients | ||
| 0.0% | ||
| Medical-therapy patients | ||
| 19.5% | ||
| 5. Torrential | ||
| Valve-replacement patients | ||
| 0.0% | ||
| Medical-therapy patients | ||
| 23.0% |
The significant reduction in regurgitation for valve-replacement patients is especially noteworthy because more than half of those patients had massive or torrential regurgitation at the beginning of the study, notes Dr. Kapadia.
“As an imaging cardiologist, I particularly appreciate the moment in the procedure when the valve is deployed,” says Rhonda Miyasaka, MD, a cardiovascular imaging specialist at Cleveland Clinic. “We literally watch the tricuspid regurgitation disappear, from one minute to the next, knowing that the patient should now feel better.”

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7d5cbade-7000-4520-ba32-dc2f47567748/Transesophageal-echocardiogram-tricuspid-valve-regurgitation)
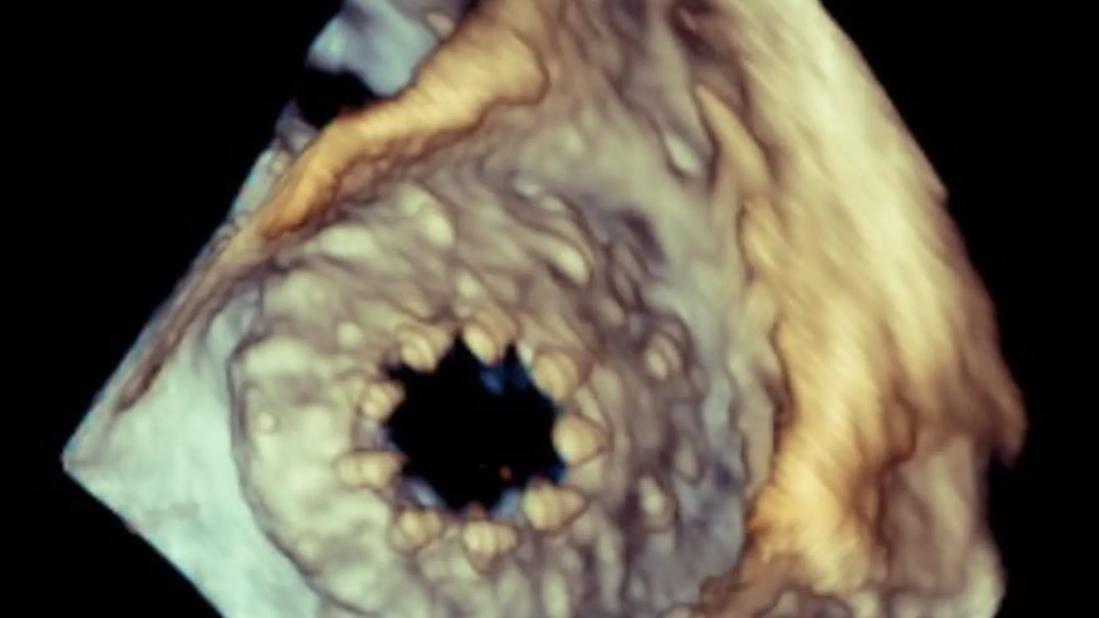
3D transesophageal echocardiography (TEE) image of a tricuspid valve with severe regurgitation.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/eb675a2f-6534-4a27-83a2-55611dea3d19/Transesophageal-echocardiogram-tricuspid-valve-replacement)
3D TEE image of the tricuspid valve after transcatheter replacement.
Although valve-replacement patients outperformed medical-therapy patients in primary outcome, they also were more likely to experience severe bleeding during the trial (15.4% vs. 5.3%) and conduction disorders leading to pacemaker implantation (17.8% vs. 2.3%) — both conditions not uncommon with valve replacement procedures and the associated anticoagulation therapy.
Patients will continue to be followed annually for five years.
“Transcatheter interventions are well established for aortic valve disease and mitral valve disease, but our study indicates the value for tricuspid valve disease as well,” Dr. Kapadia says. “Open tricuspid valve replacement is performed infrequently because patients with severe TR often have liver and kidney dysfunction as well, which increases their surgical risk. Transcatheter valve replacement could change the outlook for this patient population.”
Advertisement
The study was supported by Edwards Lifesciences.
Advertisement
Advertisement

Study supports addressing mitral regurgitation before mild tricuspid regurgitation progresses

Limited data and experience will translate to a cautious rollout
Cleveland Clinic study argues against waiting for symptoms to develop
TEER is found to be a safe and good option for severe TR in select patients
Understanding VC anatomy is critical for transcatheter tricuspid valve interventions

A scannable graphic recap of our latest data

Aortic valve replacement is best option for lowering mortality in this high-risk population
Join us in New York Dec. 6-7 for broadened version of a CME crowd-pleaser