Investigators conclude that improved self-reports go beyond possible placebo effect for severe TR
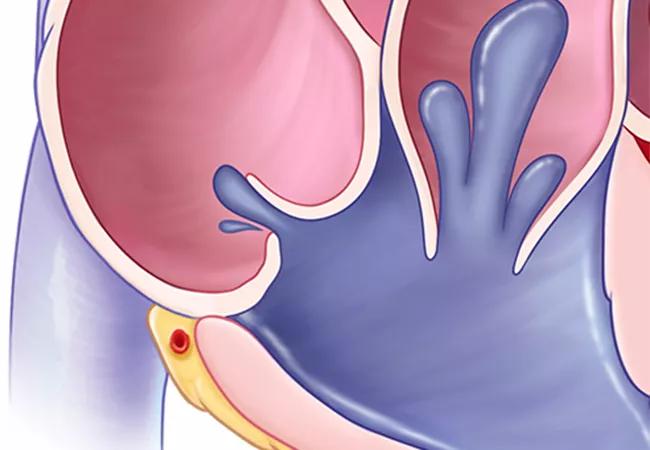
Select patients with isolated severe tricuspid regurgitation (TR) who undergo tricuspid transcatheter edge-to-edge repair (T-TEER) with the investigational TriClip Transcatheter Tricuspid Valve Repair System are likelier to be enjoying better health after a year than if they had stayed on medical therapy. So concludes a special analysis of health status data from the TRILUMINATE Pivotal Trial, reported at the 2023 Transcatheter Cardiovascular Therapeutics (TCT) meeting and simultaneously published online in the Journal of the American College of Cardiology.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“In the primary report from the TRILUMINATE Pivotal Trial earlier this year (N Engl J Med. 2023;388[20]:1833-1842), self-reported health status was the only component of the primary endpoint that significantly differed between the two unblinded treatment arms,” says study co-author Samir Kapadia, MD, Chair of Cardiovascular Medicine at Cleveland Clinic. “This caused some to question whether a placebo effect accounted for the superiority of T-TEER in the study. However, careful analysis of the evidence in this new report indicates that greater improvement in the treatment group was likely a real phenomenon.”
The TRILUMINATE Pivotal trial is an international multicenter investigation designed to compare T-TEER to medical therapy alone in patients with isolated severe TR. The primary report of one-year results showed no difference between the T-TEER and medication-only arms (175 patients in each arm) in rates of death and heart failure hospitalization. However, the composite primary endpoint favored the T-TEER arm primarily because self-reported health status ― as measured by the Kansas City Cardiomyopathy Questionnaire overall summary score (KCCQ-OS) ― showed significantly greater improvement in the T-TEER group at one year.
The KCCQ is a 23-item questionnaire covering physical limitation, symptoms, quality of life, social limitation and self-efficacy. The score ranges from 0 to 100, with a higher score indicating better health status. A score of 50 to 74 is regarded as fair to good health (equivalent to NYHA class II-III); a 10-point improvement is regarded as moderate clinical change.
Advertisement
A recognized limitation of the trial was that patients were not blinded to treatment arm, introducing the possibility of bias ― i.e., that patients who underwent T-TEER would be more likely to report improvement on the KCCQ. The current analysis sought to further examine the data to help resolve this issue and better characterize the health status benefit according to patient factors.
This analysis defined “alive and well” as patients scoring at least 60 points on the KCCQ-OS at one-year follow-up and who had no decline of 10 points or more from baseline. Overall, 74.8% of T-TEER patients reached this endpoint versus 45.9% of patients in the medication arm (P < 0.001). The number needed to treat with T-TEER to achieve this threshold was 3.5.
Difference in health status improvement between the two arms was evident at one month following randomization: the mean between-group difference in KCCQ-OS was 9.4 points higher (95% CI, 5.3-13.4) in the T-TEER group. A small additional improvement was discerned at one year, with a 10.4-point difference (95% CI, 6.3-14.6) between groups.
Within the T-TEER arm, improved KCCQ-OS scores correlated with the following:
In addition, a 10-point improvement in KCCQ-OS score at one month following T-TEER was associated with the following clinical benefits:
Advertisement
Associations between changes in KCCQ-OS score and outcomes were not apparent in the medication arm.
To help identify patients who would likely benefit from T-TEER, Dr. Kapadia notes two factors found in the analysis beyond qualification for inclusion in the TRILUMINATE study:
Dr. Kapadia lists the following points arguing against the possibility that bias accounts for the positive findings of T-TEER:
“As the KCCQ-OS assesses many symptoms of TR, it is a particularly valuable assessment of health status in this patient population,” Dr. Kapadia notes. “This analysis indicates that not only is there a real benefit to T-TEER for select patients with isolated severe TR, but that the benefit is clinically meaningful.”
Advertisement
“There has been ongoing debate about the clinical significance of demonstrating simply a significant improvement in the KCCQ score,” notes Cleveland Clinic interventional cardiologist Rishi Puri, MD, PhD, who was not involved in the study. “In a perfect world, this would require a sham-controlled study, but this is impractical. Additionally, the KCCQ has never officially been validated in the severe TR/right heart failure population. Yet in the TAVR and MitraClip populations, improvements in KCCQ score have correlated with harder clinical endpoints such as reduced heart failure hospitalizations.
“This secondary analysis is critically important to further tie the functional benefits derived from the TR-reducing procedure to significant clinical benefits,” Dr. Puri continues. “This is an endpoint that is perhaps the most meaningful to this vulnerable population who currently have no treatment options, and it should allow regulators and payers to more clearly assess the health benefits of this therapy. How regulators view these data will have a profound impact on the transcatheter tricuspid valve device field moving forward, with both replacement procedures (ortho- and heterotopic devices) and spacers in line to be evaluated against medical therapies, all of which have demonstrated similar efficacy to TriClip in single-arm studies.
“The highly select anatomical TR population represented in TRILUMINATE means that a range of transcatheter therapies will ultimately be needed in the commercial toolbox to treat the majority of patients with symptomatic severe TR,” Dr. Puri concludes.
Advertisement
The TriClip device used in the study is currently under FDA review for marketing approval. The TRILUMINATE Pivotal Trial was sponsored by Abbott, and the analysis reported here was conducted by Abbott under the direction of the academic authors.
Advertisement

Study supports addressing mitral regurgitation before mild tricuspid regurgitation progresses

A scannable graphic recap of our latest data

TRISCEND II trial reports 1-year results

Aortic valve replacement is best option for lowering mortality in this high-risk population
Join us in New York Dec. 6-7 for broadened version of a CME crowd-pleaser

Center uses advanced imaging techniques to optimize valve repair strategies

Limited data and experience will translate to a cautious rollout

Check out our latest volume and outcomes data in these key areas