Intraretinal fluid volumes and other features detectable with OCT may help predict treatment response
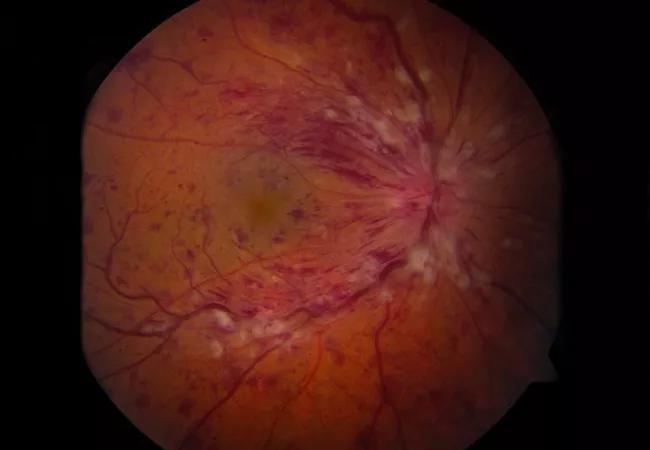
Certain optical coherence tomography (OCT) parameters may help predict treatment response in individual patients with retinal vein occlusion (RVO), suggests a study published in Eye. They also may be surrogate biomarkers of the disease’s underlying biologic pathways.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Approximately 1% of the U.S. population has RVO. It is caused by obstruction to venous drainage and can lead to various vision-threatening conditions. Macular edema is the most common of these, resulting from a flow of cytokines from injured, hypoxic tissue and leading to endothelial cell dysfunction and a breakdown of the blood-retinal barrier.
First-line therapy for macular edema secondary to RVO targets one of these cytokines, vascular endothelial growth factor (VEGF). In phase 3 trials, VEGF inhibitors including aflibercept and ranibizumab have significantly improved macular edema and visual acuity in a majority of patients with RVO. But about one in five do not respond, and approximately 40%-50% of patients do not gain three or more lines of vision with treatment.
“In RVO, the proportion of nonresponders is fairly small,” says lead investigator Justis P. Ehlers, MD, of Cleveland Clinic Cole Eye Institute. “RVO is generally a very VEGF-driven disease and often quite responsive to anti-VEGF therapy. But that can lead to some complacency in the treatment approach, making ophthalmologists too reliant on anti-VEGF alone.”
Inflammatory-based pathways also may contribute to the condition in nonresponders. To address this, a dexamethasone implant has been approved for RVO by the U.S. Food and Drug Administration (FDA). The implant provides a broad anti-inflammatory, steroid-based therapy. Also in development are numerous other therapies that may target specific pathways or provide alternative delivery strategies.
Advertisement
“A more personalized and precise approach using surrogate biomarkers to help identify the dominant underlying pathway of RVO may allow for therapy stratification based on patient profile,” says Dr. Ehlers.
In a study of 30 eyes with RVO from two previous clinical trials, aqueous cytokine expression was evaluated and was linked to specific OCT features and to anti-VEGF treatment response. Very high intraretinal fluid levels were associated with high VEGF levels, “and that’s likely someone who will respond to anti-VEGF therapy,” says Dr. Ehlers, who is Director of the Tony and Leona Campane Center for Excellence in Image-Guided Surgery and Advanced Imaging Research at the Cole Eye Institute.
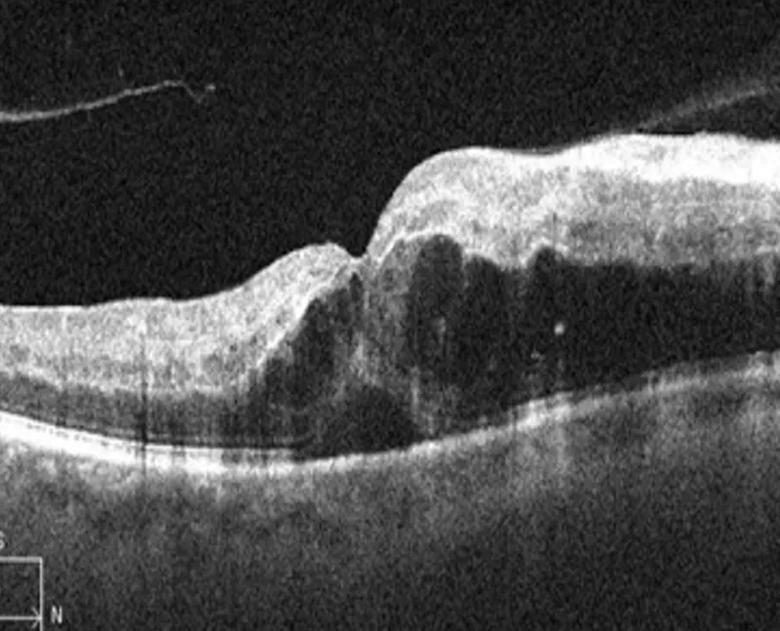
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2bfbef53-d44d-4272-b14f-6dddcb5cb69c/22-EYE-3438364-CQD-_-OCT-based-imaging-biomarkers-inset_jpg)
OCT image of a retinal vein occlusion demonstrating significant intraretinal fluid. Using advanced analysis technology, assessment of fluid volume and other image features may provide insights into underlying biology and the likelihood of response to therapy. For example, high volume of intraretinal fluid is associated with high VEGF expression and good response to anti-VEGF therapy.
With more moderate fluid levels, where the correlation with VEGF was somewhat weaker, anti-VEGF therapy is still first line. However, “if you treat once and you don’t get a response, those are eyes you might want to change much sooner to other therapies, such as intravitreal triamcinolone or dexamethasone implants. This reinforces that nonresponders probably have another pathway that is driving the macular edema and disease pathophysiology, or at least driving it concurrently with the VEGF pathway,” he says.
In the investigator-initiated IMAGINE study co-designed at Cleveland Clinic, macular OCT scans underwent automated analysis to generate intraretinal and subretinal fluid extraction metrics as well as segmentation of multiple retinal layers, including assessing ellipsoid zone integrity (i.e., photoreceptor integrity surrogate). In addition, samples of aqueous humor were obtained at baseline (before the patients received anti-VEGF injections) and at four and nine months following treatment.
Advertisement
Based on OCT profiles, 21 eyes were identified as treatment responders and five as nonresponders. Of the 21 responders, 13 were defined as rebounders based on a 50% increase in central subfield thickness after initial improvement with therapy.
VEGF levels directly correlated with intraretinal fluid volume, while angiogenin was inversely correlated with fluid indices. Multiple cytokines were directly correlated with ellipsoid zone disruption.
Baseline VEGF levels were significantly higher in all responders compared to nonresponders (P = .02). Conversely, nonresponders tended to have lower levels of VEGF and higher levels of epidermal growth factor, suggesting a potentially more inflammation-driven process.
Rebounders tended to have significantly decreased baseline levels of two cytokines, angiogenin and TIMP-1 (P = .019 and P = .015, respectively).
There’s more ongoing work in this field beyond currently approved therapies. The FDA recently approved the drug Vabysmo® (faricimab-svoa) for the treatment of wet age-related macular degeneration and diabetic macular edema. This drug targets both VEGF and angiopoietin-2. Phase 3 trials of the agent in RVO demonstrated favorable top-line results.
“As more therapies are approved with additional targeted actions, nonresponders in this condition will be a focus area to evaluate a more broad therapeutic action,” says Dr. Ehlers. “Several earlier-stage therapeutics for RVO also are being evaluated in clinical trials.”
The IMAGINE study was initial proof of concept. In an ongoing prospective multicenter clinical trial, Dr. Ehlers and colleagues are gathering aqueous fluid from eyes and comparing steroid treatment to anti-VEGF therapy to see whether those baseline cytokines combined with imaging biomarkers could have been used to predict which patients would have been better off with one therapy for diabetic macular edema versus another.
Advertisement
“Hopefully that will give us more information on the underlying connection between imaging features and cytokine expression,” he says. “However, since extracting fluid from eyes for cytokine analysis isn’t currently practical outside of clinical trials, our goal is to identify imaging biomarkers that could be surrogates for these cytokines and pathways. A noninvasive marker could be highly valuable.”
Advertisement
Advertisement

Innovative approach to the procedure can yield significant relief in complex cases

A scannable recap of our latest data in these clinical areas

Site visits offer firsthand lessons in clinical and operational excellence in cardiovascular care

The advanced stage of diabetic retinopathy is among the most challenging for retinal surgeons

New insights on effectiveness in patients previously treated with other anti-VEGF drugs
Study identifies factors that may predict vision outcomes in diabetic macular edema

Watch for sudden unilateral vision loss without pain

Prematurity is linked with poorer outcomes of retinal surgery in adulthood