Successful outcome with no harm to mother or newborn
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The hypercoagulability of pregnant women increases their risk for thromboembolic diseases. Although fibrinolytic therapy has been used to treat this population for some conditions, clinical trials have not evaluated its use in managing parapneumonic pleural effusions. Our case — presented in the Journal of Thoracic Disease — is the first reported use of tissue plasminogen activator (tPA) to treat a pregnant patient for a complicated parapneumonic pleural effusion.
Studies evaluating the effectiveness of intrapleural (IP) fibrinolytics in treating parapneumonic pleural effusions are inconclusive. Some studies fail to show a reduced need for surgical interventions when IP fibrinolytics are initially administered. However, a 2014 meta-analysis demonstrated that IP fibrinolytics can be effective in reducing the need for surgical intervention and decreasing the length of hospital stays. Systemic side effects of the drug include fatigue, leukocytosis, bleeding and fever.
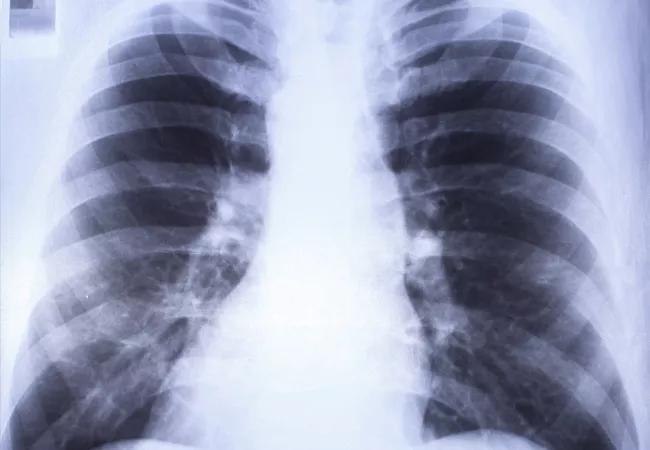
A 35-year-old female at 32 weeks of gestation was admitted to the hospital after five days of cough, dyspnea and right-sided pleuritic chest pain. Oral antibiotics had not resolved the condition. The patient’s chest radiograph showed right-sided pulmonary infiltrates. A chest CT showed no pulmonary embolism, but did reveal right lower lobe consolidation and an ipsilateral loculated pleural effusion. An ultrasound indicated the pleural effusion measured 14 cm x 9.5 cm x 6.1 cm with a volume of approximately 811 mL.
Advertisement
Pleural fluid studies showed: pH 7.51, glucose 12 mg/dl, LDH 3619 U/L and total protein 5 g/dl. Pleural fluid cultures were negative. This indicated a complicated parapneumonic pleural effusion. Her rapid influenza test with throat swab was positive for influenza A. Sputum culture grew Group A Streptococcus.
The patient was treated for influenza pneumonia with superimposed bacterial pneumonia using oseltamivir and penicillin G. The pleural effusion was initially treated by percutaneous placement of an intercostal chest tube. Initial drainage was 300 mL of nonpurulent straw-colored fluid. Repeat imaging studies showed that the pleural effusion remained substantial. The use of IP tPA was considered to increase output from the chest tube. The patient was informed of the potential risks, which included chest pain, placental bleeding, hemorrhage and IP hematoma. With the patient’s consent, 2.5 mg of tPA was administered via the chest tube. The tPA was left in the pleural space for two hours while the patient was turned from side to side every 30 minutes. Following the two hour dwell time, the chest tube was placed on suction at -20 cmH2O. This resulted in drainage of additional 250 mL of pleural fluid.
Chest radiographs and ultrasound demonstrated a significant decrease in the size of the pleural effusion, and the patient did not require additional IP tPA. Four days after tPA treatment, the chest tube was removed. Subsequent cultures from the pleural fluid were negative. The patient was released from the hospital and completed two weeks of amoxicillin/clavulanic acid.
Advertisement
The patient had an uneventful delivery at 40 weeks gestation, and the newborn had no adverse sequelae.
All randomized controlled trials assessing the use of tPA for treating thrombolysis have excluded pregnant women, and data related to its use in this population come from case reports. There are no reported cases of teratogenicity or an increase in fetal risk when systemic tPA is used during pregnancy. All reported instances of fetal or maternal deaths were caused by the severity of the patient’s condition rather than the use of thrombolytics.
The FDA categorizes alteplase and other tissue plasminogen activators as pregnancy category C medications. However, drugs with a molecular weight greater than 1,000 Da are unlikely to cross into the placenta, and tPA has a molecular weight of 7,200 Da. The drug’s half-life is four to five minutes. It is metabolized hepatically, and after 20 minutes, only a 10 percent concentration remains.
Our patient was late in the third trimester of her pregnancy, and this form of treatment spared her the potential morbidity of more invasive surgical procedures. Overall, the decision to use IP tPA in a pregnant patient with a parapneumonic pleural effusion should be made with careful consideration of the risks to both the patient and fetus.
Dr. Choi is staff in the Departments of Pulmonary Medicine and Critical Care Medicine.
Advertisement
Advertisement

Takeaways from the most recent annual meeting centered around clinical advances, AI integration and professional development

Recent breakthroughs have brought attention to a previously overlooked condition

A review of treatment options for patients who may not qualify for surgery

Looking at the real-world impact and the future pipeline of targeted therapies

The progressive training program aims to help clinicians improve patient care

New breakthroughs are shaping the future of COPD management and offering hope for challenging cases

Exploring the impact of chronic cough from daily life to innovative medical solutions

How Cleveland Clinic transformed a single ultrasound machine into a cutting-edge, hospital-wide POCUS program