Hysteroscopy used to make rare diagnosis
A 22 year-old G2P1011, who had undergone a first-trimester termination 9 weeks previously, presented to the Emergency Department with heavy vaginal bleeding.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The patient’s urine was negative for human chorionic gonadotropin (hCG). Ultrasound revealed a 2.6-cm vascular mass in the endometrial canal, which was initially thought to be a retained product of conception (POC). Also considered in the differential diagnosis were enhanced myometrial vascularity (EMV) (previously known as arteriovenous malformation), myoma and various tumors. Prior uterine instrumentation or surgery, such as curettage, cesarean section or myomectomy, is the major acquired risk factor for EMV. It often occurs in the context of pregnancy, but also in patients with endometrial or cervical carcinoma, gestational trophoblastic disease and cesarean scar pregnancy.
The patient’s ultrasound failed to mention the peak systolic volume (PSV), which was critical in this case, because PSV > 20 cm/sec in a high-flow vascular lesion such as this one is strongly suggestive of EMV.
The patient continued to bleed heavily. When her hemoglobin dropped to 6.9 g/dL from 8.3 g/dL overnight, she received a transfusion with 2 units of packed red blood cells. Hysteroscopy and possible uterine curettage were scheduled.
On hysteroscopy, a bluish mass measuring approximately 2 cm was revealed.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/39cd9da5-263a-47b2-a764-2ed81632095c/20-WHI-1898320-CQD_Bradley_Hysteroscopy_650x450_226_jpg)
A third-year resident in the ER noticed another hallmark of EMV: uterine pulsation in sync with the patient’s heartbeat. He immediately shared an intraoperative photo with Cleveland Clinic’s Linda Bradley, MD, Professor of Ob/Gyn and Reproductive Biology, Medical Director of the American Association of Gynecologic Laparoscopists (AAGL). “His education paid off because we were able to stop the procedure,” she says. “Had the lesion been biopsied, the patient could have experienced massive hemorrhage.”
Advertisement
To confirm the diagnosis, magnetic resonance imaging and magnetic resonance angiography (MRA) were performed. Early post contrast enhancements were visible in the arterial phase. Tubular flow was seen along the right lateral fundal myometrium on T2-weighted images, which formed a 2.7-cm mass-like projection into the right anterolateral myometrium. The early enhancement in the arterial phase with asymmetric filling of the right gonadal vein, seen on post-contrast coronal images, was clinically consistent with EMV.
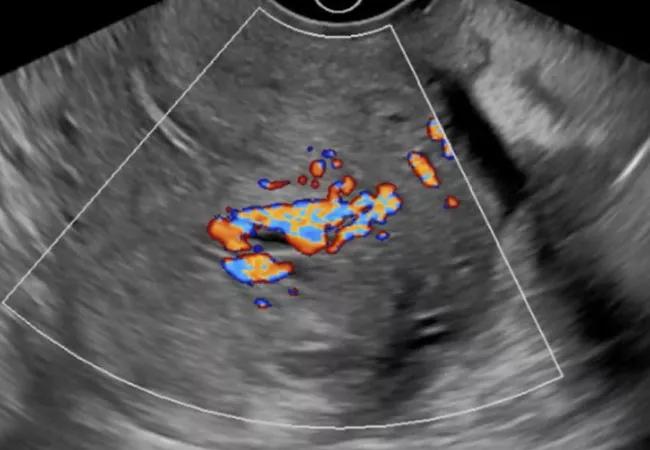
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/cbe5dda9-0996-41a5-ab92-480c266c5ad4/20-WHI-1898320-CQD_Bradley_Hysteroscopy_650x450_104_jpg)
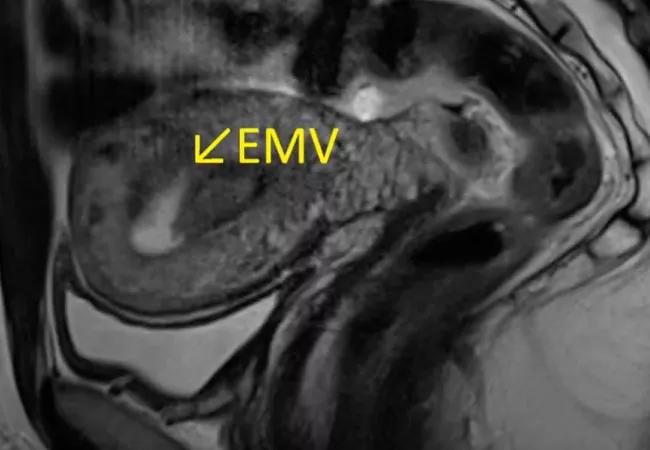
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/d5deae81-c50a-42e1-be91-0dc2c961c089/20-WHI-1898320-CQD_Bradley_Hysteroscopy_650x450_342_jpg)
With confirmation of the EMV diagnosis, embolization of the lesion was performed, after the patient was counseled about the potential for decreased ovarian reserve, infertility, uterine synechiae and pregnancy-related complications. Angiography once again confirmed the EMV diagnosis, with a finding of bilaterally enlarged, tortuous uterine arteries perfusing the hypervascular mass. To slow arterial flow, each uterine artery was embolized with 500 to 700 tris-acryl gelatin microspheres.
After embolization, branches of the EMV that had been visible on angiography of the main uterine arteries could no longer be seen. The patient was discharged on day three post-procedure. Follow-up hysteroscopy performed two weeks later showed a 2-cm raised area of white tissue on the posterior uterus with no pulsation and no intrauterine lesions. A few loose microspheres also were seen in the uterine cavity. By six weeks post-embolization, the patient was no longer bleeding, and on hysteroscopy, a 0.5-cm posterior nodule was visible. It was avascular, with a circumferential pseudodecidual reaction.
Advertisement
Inadvertent vascular injury or bleeding is a risk with any uterine procedure. In this case, the risk of significant hemorrhage was increased because the patient’s lesion involved a high-flow vascular system. However, because there was a concern about possible retained POC, hysteroscopy was appropriate. Risk of injury during hysteroscopy can be minimized by using cervical dilators only to the level of the internal cervical os. Direct trauma can be limited by advancing the hysteroscope into the uterine cavity under direct visual guidance. An infusion pump should be used to vary the intrauterine pressure and maintain a clear field while avoiding excess fluid absorption. Blind curettage should be avoided.
“Uterine EMV is rare but the outcomes can be disastrous if it’s not considered in patients with uterine bleeding,” says Dr. Bradley. “As demonstrated in our patient, bleeding post-termination isn’t always caused by retained products of conception.”
Consultation with interventional radiology is recommended for patients with EMV who have significant bleeding. Embolization generally is the treatment of choice. Conservative management may be successful when ultrasound reveals a vascular-appearing lesion, the PSV is high, and the patient is stable. Case reports also exist of use of hysteroscopic electrosurgical coagulation of the lesions of EMV. Rarely is surgery or hysterectomy required to control vessels supplying the mass.
The other take-home message from this case, says Dr. Bradley, is the importance of thorough history-taking. “Knowledge frames how clinicians behave. We can be led down the wrong path if a woman is too embarrassed to disclose a key piece of information. Take time to reassure patients about confidentiality so that you can get a good history.”
Advertisement
Advertisement

Uterine transposition cleared the field for radiation therapy

ACOG-informed guidance considers mothers and babies

Prolapse surgery need not automatically mean hysterectomy

Artesunate ointment shows promise as a non-surgical alternative

New guidelines update recommendations

Two blood tests improve risk in assessment after ovarian ultrasound

Recent research underscores association between BV and sexual activity

Psychological care can be a crucial component of medical treatment