Why PML continues to elude risk assessment strategies
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Rheumatologists and other physicians who care for patients with Immune Mediated Immunoinflammatory Diseases (IMIDS) consider safety as primary in the shared and informed decision making process of selecting immunosuppressives and, in particular, biologic therapeutics. Our field has developed many strategies to assess risk over the past two decades, including the use of scoring systems like the RABIT risk score. RABIT allows clinicians to numerically estimate the risks of serious infectious complications.
Another strategy to mitigate risk has included screening prior to biologic therapies for infections such as tuberculosis and hepatitis B and C. In some situations prophylactic antimicrobials (i.e. prevention of PJP) have been effective.
Each of these strategies has added to the margins of safety within which we operate. But some infections have eluded these new margins of safety and remain a significant risk. One of the most feared is the development of progressive multifocal leukoencephelopathy (PML) secondary to the reactivation of latent infection with the human papilloma virus known as JC. Not only have risk mitigation strategies, such as useful biomarkers, been elusive, but the condition itself is often deadly and largely untreatable.
Concerns about PML, a disease that is genuinely rare, only came to light with the HIV epidemic in the precombination antiretroviral era. PML became a reality for patients with IMIDS in 2005, when several seemingly rare and unusual case reports surfaced in conjunction with the introduction of the anti-adhesion monoclonal natalizumab.
Advertisement
Since those original case reports, there have been many hundreds of cases associated with this therapy, and a risk mitigation strategy has been developed combining serologic testing and clinical surveillance for patients with multiple sclerosis (MS). PML is observed exclusively (with rare exceptions) in immunosuppressed patients and can be difficult to diagnose. Neurologic signs and symptoms may be subtle initially, and in patients with rheumatic disease, such new onset symptomatology are often thought to be complications of the underlying systemic autoimmune disease.
Prompt neuroimaging followed by CSF analysis for the JC virus detected by PCR and are essential. Less commonly, brain biopsy may be needed to secure the diagnosis. Given the gravity and complexity of PML, many clinicians and patients are fearful of this complication with immunosuppression, and until recently, we lacked a cohesive risk analysis for drug-induced (or immunosuppression-associated) PML.
In 2015, we published a comprehensive risk-based assessment for PML, classifying many of the drugs used to treat IMIDS based on PML risk, to better inform clinician decision making. The common misconception that risks are high with all immunotherapies prompted us to undertake this analysis. Also missing from the dialogue has been the increased risk caused by certain underlying conditions such as systemic lupus erythematosus.
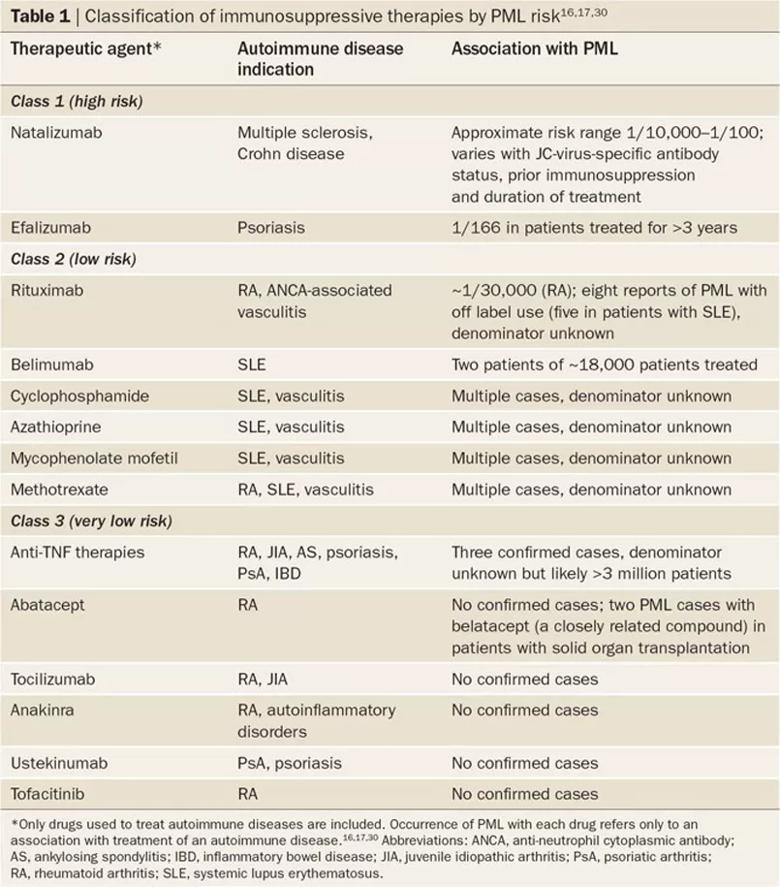
image caption: classification of immunosuppressive therapies by PML risk. First published here, republished with permission from authors.
Advertisement
Of note, most IMIDS (with the exception of MS) are treated with drugs in Class 2 or Class 3, and for these drugs, the risk of PML is low. Based upon these data, there is no clinical indication to screen with JC antibodies prior to administering drugs in Class 2 and Class 3.
At the upcoming Biologic Therapies Summit VII, held April 6-8, 2017, at the Intercontinental Hotel and Conference Center in Cleveland, Ohio, Kevin Winthrop, MD, MPH, and Jack Cush, MD, will lead a half-day session dedicated to comorbidities including serious infections.
This year’s Summit will also highlight the hopes and challenges of precision medicine for the nearly 50 million people in the U.S. with disorders of immunity. For registration and additional information on the Summit, visit www.ccfcme.org/biotherapiesVII.
This activity has been approved for AMA PRA Category 1 Credit™.
Dr. Calabrese is Director of the R.J. Fasenmyer Center for Clinical Immunology in the Department of Rheumatic and Immunologic Diseases.
Advertisement
Advertisement

The case for continued vigilance, counseling and antivirals

High fevers, diffuse rashes pointed to an unexpected diagnosis

No-cost learning and CME credit are part of this webcast series

Summit broadens understanding of new therapies and disease management

Program empowers users with PsA to take charge of their mental well being

Nitric oxide plays a key role in vascular physiology

CAR T-cell therapy may offer reason for optimism that those with SLE can experience improvement in quality of life.

Unraveling the TNFA receptor 2/dendritic cell axis