Genetic polymorphisms and response to TNFα blockers
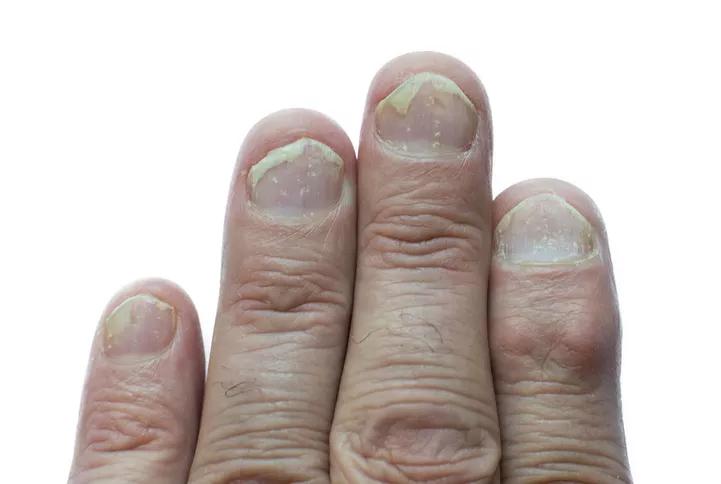
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/fac6b2ae-7340-4709-b54c-15bb2a60efd8/GettyImages-855985394_jpg)
psoriasis of the fingernails and psoriatic arthritis on white background
By M. Elaine Husni, MD, MPH, James K. Sullivan, BA, and Unni M. Chandrasekharan, PhD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
As we improve our understanding of the pathogenesis of psoriatic diseases, our treatment management can be refined as well. Our hope is to move away from the “one size fits all” approach and begin a new era of a more individual approach to treatment. Over the course of 20 years, and according to the ACR/NPF guidelines,1 tumor necrosis factor alpha (TNFα) blocking agents have become first-line treatment for patients with moderate to severe psoriatic arthritis (PsA).
Research supports TNFα blockers (TNFi therapy) as safe and effective in relieving a wide spectrum of symptoms and inhibiting PsA-related joint damage. For 40%-50% of patients2 with PsA, however, TNFα blockers work insufficiently, lose efficacy over time or are not effective at all, and no clinical test exists to predict who will or won’t benefit from TNFα blocking therapy.
At Cleveland Clinic’s Orthopaedic & Rheumatologic Institute, we are studying genetic polymorphisms for clues that we hope will lead to improved understanding of why some individuals do not respond to TNFα blockers, more treatment predictability and, ultimately, allow for a personalized therapeutic approach for those with PsA.
PsA is a seronegative inflammatory arthritis that affects up to 30% of patients with psoriasis and 0.25% of the United States population. It can be debilitating, with symptoms that include pain and swelling in the joints (commonly hands, feet, wrists, ankles and knees) and lower back; reduced range of motion; inflammation of the entheses, and dactylitis. Dermatologic symptoms commonly include scaly areas on the scalp, elbows, knees and lower spine; papules on the arms, legs and torso; and pitting or detachment of fingernails and toenails.
Advertisement
PsA also can significantly reduce quality of life and increase overall risk of mortality in younger patients compared to the general population. Increased risk of cardiovascular disease is the largest contributor to mortality in this population,3 and currently available assessment tools such as the Framingham Risk Score underestimate cardiovascular risks for patients with PsA.
Polymorphisms in the TNFα receptor 2 (TNFR2) gene have long been implicated in the pathogenesis and response to treatment for immune-mediated diseases, including rheumatoid arthritis, inflammatory bowel disease, ankylosing spondylitis and psoriasis.4,5 There are limited studies on TNFR2 polymorphisms in PsA, and conclusions are not definitive, as the sample sizes are small and the definition of TNFi response is unclear. In a limited sample of patients with PsA, preliminary data from the Husni laboratory at Cleveland Clinic identified a relationship between a particular TNFR2 gene polymorphism (rs1061622) and response to anti-TNFα therapy. The rs1061622 represents T/G polymorphisms at Based on our preliminary results, we hypothesize that adults with PsA who have TNFR2 M196R (minor variant) show a poor response to anti-TNFα therapy and dysregulated lipid profiles compared to those without the M196R variant because of constitutive pro-inflammatory activity of TNFR2 M196R.
Our study goal is to establish the mechanistic relationships of these genetic markers to anti-TNFα therapy. We will accomplish this by accessing Cleveland Clinic’s Psoriatic Disease Biorepository and determine the following:
Advertisement
• The impact of a specific polymorphism (rs1061622) on anti-TNFα therapy response.
• Differences in signaling pathways by which rs1061622 polymorphism (T allele versus G allele) modulates response to TNFα inhibition.
• The association of rs1061622 polymorphism with serum lipid profiles of patients with PsA.
If data support the predictability of poor response to TNFα therapies in certain patients with TNFR2 polymorphisms, an inexpensive and efficient test could be performed on any patient with PsA.
Defining predictability has the potential to risk-stratify those PsA patients who may benefit from TNFi therapy versus those who would not benefit from TNFi therapy. This would reduce considerable suffering and unnecessary expense in those with PsA who may need to cycle through many different medications for relief. Trials of TNFα blocker(s) therapy require up to three months to demonstrate efficacy, during which patients may require time off for either IV infusions performed at infusion center or need teaching visits for self-injections.
In cases of treatment failure, patients continue to experience symptoms, and may face another three-month cycle of different medication – without indication of whether it will work better than the first.
Those for whom anti-TNFα treatment fails have limited options, although new drugs are approved almost every year. Therapies that could be introduced after anti-TNFα failures include anti-interleukin 23 medications such as guselkumab (Tremfya®), risankizumab (Skyrizi®) and ustekinumab (Stelara®); anti-interleukin 17 medications such as secukinumab (Cosentyx®) and ixekizumab (Taltz®); as well as in combination with other oral DMARDs such as methotrexate.
Advertisement
Similarly, identification of an association of rs1061622 polymorphism with dysregulated lipid metabolism may help to identify PsA patients at risk of related cardiovascular disease and this may lead to reduce mortality, if intervened early.
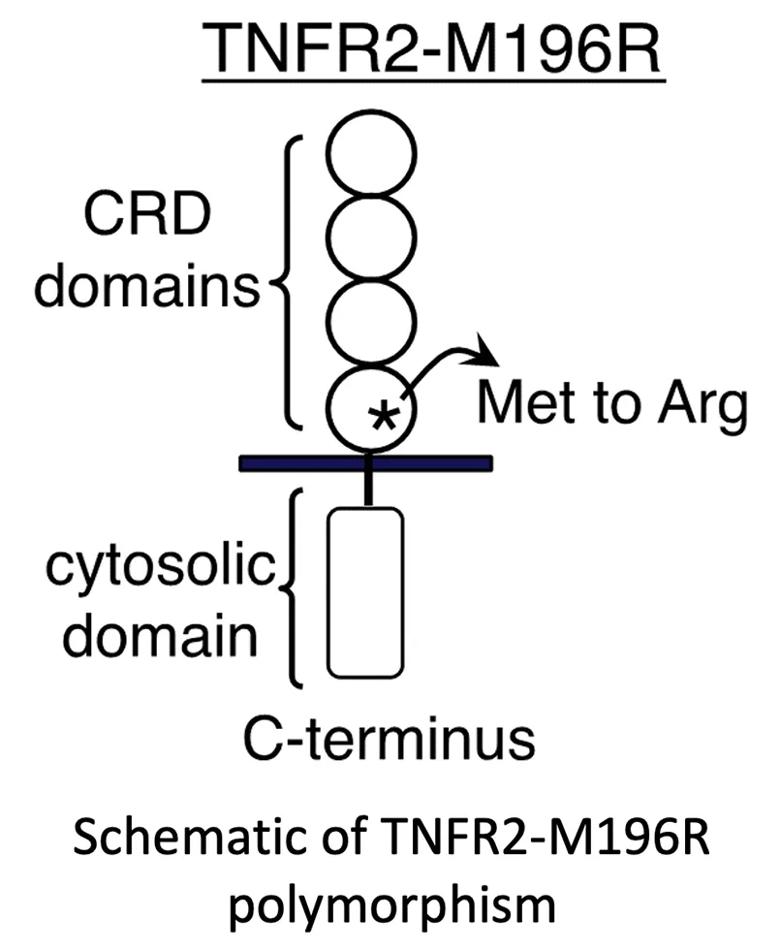
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/0fa12cd4-c91a-47b7-ac66-e9b78256b4a3/22-RHE-3431508-CQD-Inset_jpg)
References
1. Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, Dubreuil M, Dunham J, Husni ME, et al. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Rheumatol. 2019 Jan;71(1):5-32. doi: 10.1002/art.40726. Epub 2018 Nov 30.
2. Brahe, C. H. et al. Retention and response rates in 14 261 PsA patients starting TNF inhibitor treatment-results from 12 countries in EuroSpA. Rheumatol. Oxf. Engl. 59, 1640–1650 (2020).
3. Ogdie, A. et al. Comprehensive Treatment of Psoriatic Arthritis: Managing Comorbidities and Extraarticular Manifestations. J. Rheumatol. 41, 2315–2322 (2014).
4. Gwan Gyu Song, Sang-Cheol Bae, Young Ho Lee. Associations between functional TNFR2 196 M/R polymorphisms and susceptibility to rheumatoid arthritis: a meta-analysis. Rheumatol Int. 2014 Nov;34(11):1529-37.
5. Yan Hong and Ruiquan Wang. Int J Clin Exp Pathol 2016;9(11):11936-11943.
6. Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–1196.
7. Benjafi eld AV, Wang XL, Morris BJ. Tumor necrosis factor receptor 2 gene (TNFRSF1B) in genetic basis of coronary artery disease. J Mol Med (Berl). 2001 Apr;79(2-3):109-115.
Advertisement
Advertisement
Researching the biological basis for why treatment is or is not effective
Scribing system helps create more face-to-face interactions
A conversation with Leonard Calabrese, DO
The case for continued vigilance, counseling and antivirals
High fevers, diffuse rashes pointed to an unexpected diagnosis
No-cost learning and CME credit are part of this webcast series
Summit broadens understanding of new therapies and disease management
Program empowers users with PsA to take charge of their mental well being