First reported case in a patient with diffuse cutaneous systemic sclerosis
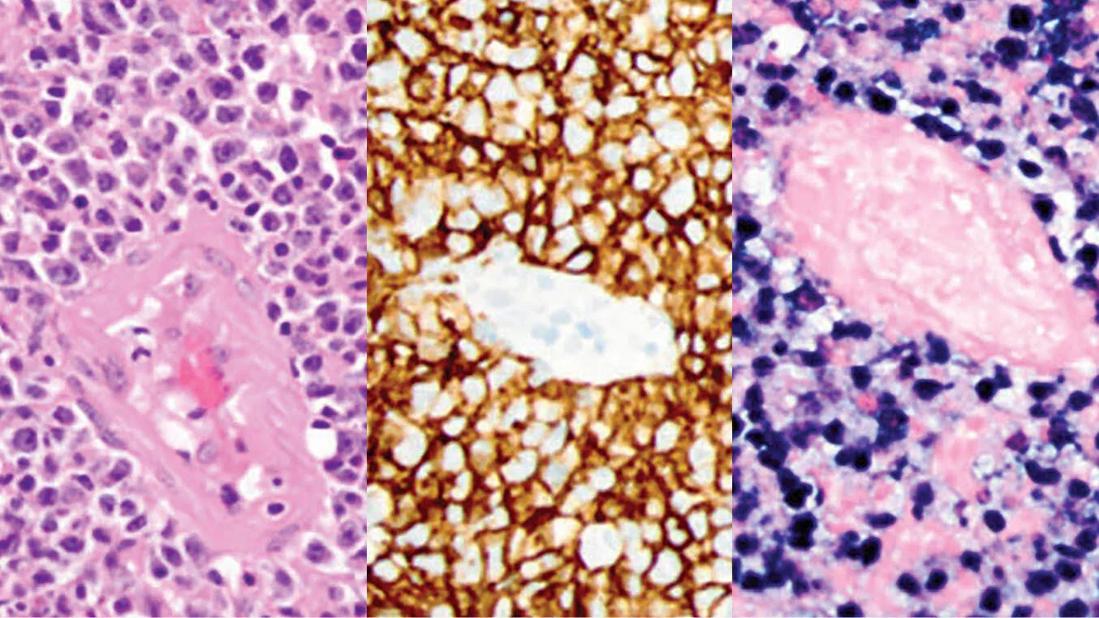
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/dd17121e-205a-41b7-9bb4-74980cdea6f0/22-RHE-3091667-Chatterjee-EBV-hero-jk-1_jpg)
EBV-Associated Lymphoma
From left, hematoxylin and eosin stain shows atypical perivascular cells; the cells are positive for CD20 by immunohistochemistry; the cells are chromogenic in situ hybridization for EBV-encoded RNA positive.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
By Soumya Chatterjee, MD, MS, FRCP
A 46-year-old woman presented to Cleveland Clinic with Raynaud phenomenon, sclerodactyly, rapidly progressive skin tightness, polyarthralgia with morning stiffness, proximal myopathy, dry eyes and mouth, and gastroesophageal reflux disease.
Laboratory testing showed positive antinuclear antibody and positive anti-RNA polymerase III antibody. Her anti-SS-A and anti-SS-B antibodies were negative. Her blood count, comprehensive metabolic panel and inflammatory markers were normal. A pulmonary function test and 2D echocardiogram also were normal.
Based on these findings, she was diagnosed with diffuse cutaneous systemic sclerosis (dcSSc) with secondary Sjögren syndrome.
Her modified Rodnan skin score (mRSS), a measure of skin tightness, was 17/51 at initial presentation and rapidly increased to 27/51 within eight months. She also developed severe pruritus and progressive joint contractures.
She was started on 2 g daily mycophenolate mofetil (MMF), which effectively slows down progressive skin tightness in dcSSc. MMF has become the first-line immunosuppressive therapy for interstitial lung disease (ILD) in scleroderma and also the first-line therapy used by many scleroderma experts to treat diffuse and rapidly increasing skin tightness in dcSSc.
After eight months of treatment, she had an mRSS of 8/51, a remarkable regression. Her widespread skin tightness diminished from her face, extremities and trunk to just her fingers. In addition, her joint pains, contractures, proximal myopathy, pruritus and fatigue improved substantially. We advised her to continue MMF 2,000 mg daily to maintain her improved mRSS. In addition, her Raynaud phenomenon was well controlled on amlodipine 2.5 mg daily; she took omeprazole for acid reflux disease and pilocarpine tablets for her dry mouth.
Advertisement
For five years, the patient’s condition was stable. She then developed intermittent right-sided headaches similar to her previous migraines. She soon presented to her local emergency room with an onset of neurological symptoms: more severe headaches, nausea, vomiting, gait instability, lightheadedness, blurry vision (worse on leftward gaze) and word-finding difficulty. Physical examination showed left homonymous hemianopia.
She underwent an MRI of her brain, revealing a single 2.4-cm peripherally enhancing right parietal lobe lesion with centrally restricted diffusion and significant adjacent vasogenic edema with local mass effect. Chest CT showed a few indeterminate subcentimeter pulmonary nodules and mild interstitial fibrosis. CT of the abdomen and chest was normal.
A biopsy of the right parietal lobe mass was performed and confirmed a diagnosis of diffuse large B-cell lymphoma associated with Epstein-Barr virus (EBV). As a result, her MMF treatment was discontinued.
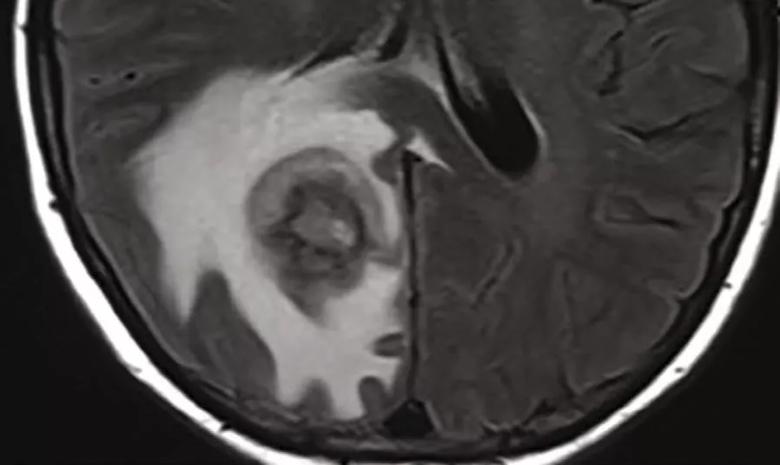
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2f0bd2b7-c02b-4f12-b80e-976948f318f9/22-RHE_3091667_Chatterjee_Inset_jpg)
An MRI scan of the brain revealed a solitary 2.4 cm peripherally enhancing right parietal lobe lesion with centrally restricted diffusion and significant adjacent vasogenic edema and local mass effect.
This is the first report of EBV-associated primary central nervous system lymphoma (ePCNSL) in a dcSSc patient receiving long-term MMF treatment. ePCNSL has been increasingly reported in patients receiving immunosuppressive agents following organ transplants or for autoimmune diseases. In addition to MMF, other immunosuppressive agents associated with development of ePCNSL include methotrexate, azathioprine and cyclophosphamide. This case raises concerns about the safety of MMF. A safe dose and duration of long-term therapy with MMF are unknown. In the ePCNSL cases reported so far, the duration of MMF treatment ranged from one to five years. At the rheumatology clinic, we are vigilant in monitoring patients on long-term MMF for signs of ePCNSL, including focal and nonfocal neurological deficits, so that they can be recognized and treated promptly to prevent permanent neurological deficits. We also consider stopping MMF after three to four years of continuous treatment if no additional benefit is anticipated.
Advertisement
Advertisement
Researching the biological basis for why treatment is or is not effective
Scribing system helps create more face-to-face interactions
A conversation with Leonard Calabrese, DO
The case for continued vigilance, counseling and antivirals
High fevers, diffuse rashes pointed to an unexpected diagnosis
No-cost learning and CME credit are part of this webcast series
Summit broadens understanding of new therapies and disease management
Program empowers users with PsA to take charge of their mental well being