Majority of patients achieve complete pathologic response after four-dose cemiplimab therapy
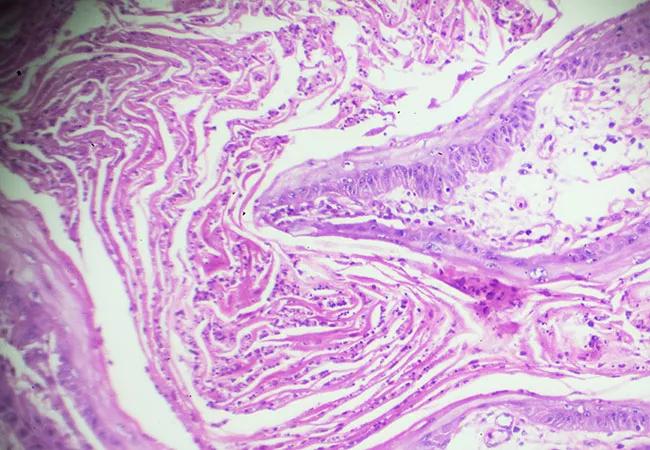
51% of patients with resectable cutaneous squamous-cell carcinoma achieved a pathological complete response and 13% achieved a pathological major response to the anti–programmed cell death 1 (PD-1) monoclonal antibody cemiplimab, according to recent findings published in the New England Journal of Medicine.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“The most provocative takeaway was that after four dose of the treatment, half of the patients had a complete pathologic response,” says Jessica Geiger, MD, study co-author and medical oncologist specializing in head and neck and skin cancers at Cleveland Clinic. “The study highlights the importance that we may want to consider immunotherapy prior to moving to surgery or in place of surgery or radiation for a great deal of the patient population.”
Though it has long been known that the medication is effective for recurrent or metastatic cutaneous squamous cell carcinoma that is not amenable to surgery or radiation therapy, a confirmatory study was needed to validate its efficacy for resectable cutaneous squamous-cell carcinoma. If found early, the disease has historically been treated successfully with surgery alone, yet a small percentage of patients with advanced disease or adverse histopathological features may require adjuvant radiation therapy and possibly systemic therapy in addition to surgery.
Cemiplimab has been approved for treating metastatic or locally advanced cutaneous squamous-cell carcinoma for which there is no curative local treatment option. In this Phase 2, multicenter, nonrandomized study, researchers evaluated cemiplimab as neoadjuvant therapy in patients with resectable stage II, III or IV cutaneous squamous cell carcinoma. Patients received cemiplimab, administered at a dose of 350 mg every three weeks for up to four doses, before undergoing surgery with curative intent.
Advertisement
A total of 79 patients were enrolled and received neoadjuvant cemiplimab. An independent review found 40 patients (51%) achieved a pathological complete response and 10 patients (13%) achieved a pathological major response after four doses. Though adverse events occurred in 87% of patients, they were mostly very low grade. In the study (NCT04154943), funded by Regeneron Pharmaceuticals and Sanofi, Grade 3 or higher adverse events were observed in 18% of patients.
After surgery, it was at the investigator’s discretion whether to continue immunotherapy for a year, perform surgery or observe the patient. For the investigators at Cleveland Clinic, they opted to observe the patients who had achieved a complete pathological response.
The primary endpoint of the study was pathological complete response where there is no viable disease visible under the microscope. “In time, we’ll be able to report whether these patients required any other cancer-directed therapy in der to maintain no evidence of disease,” says Dr. Geiger.
Cemiplimab was originally approved in 2018 for treatment of cutaneous squamous cell carcinoma for which radiation or surgery was infeasible. “My colleagues and I across the country now have a large collection of clinical experience of patients who are older, have large lesions or have comorbidities for whom surgery or radiation is too risky,” says Dr. Geiger. “Now we’re seeing that immunotherapy could be an alternative to surgery and radiation and is a treatment many patients tolerate well.”
Advertisement
Understanding who will and who will not respond to this treatment remains a big question. “We need a way to identify patients for whom immunotherapy is effective and who may not need surgery at all,” said Dr. Geiger. “And equally important, we need to identify patients who are not likely to respond to immunotherapy and who are technically resectable, to avoid delays of a potentially curative surgical approach. That’s the difficult part. What is reassuring is that there is a 50% chance that with just a few doses of immunotherapy, many patients can likely be cured.”
Dr. Geiger and a multidisciplinary team of medical oncologists, surgeons, radiation oncologists and dermatologists serve on a tumor board to review cases of recurrent or aggressive disease of the head and neck. “I’m already seeing patients in clinic where we’re able to recommend this therapy instead of moving straight to surgery.
Advertisement
Advertisement

Pembrolizumab does not improve outcomes, but immunotherapy may still offer benefit

Major study demonstrates importance of having a multidisciplinary approach to treatment for large, locally advanced tumors

Identifying candidates for further screening and treatment

Satellitosis or in-transit metastasis is a rare yet significant risk factor for this skin cancer that should be reflected in clinical staging systems.
Patient able to avoid surgery and radiation despite having rapidly growing mass

Benefits of neoadjuvant immunotherapy reflect emerging standard of care

Phase 2 trials investigate sitagliptin and methimazole as adjuvant therapies

Why Cleveland Clinic is launching its cardioimmunology center