Collaboration was key to identifying source of nerve condition
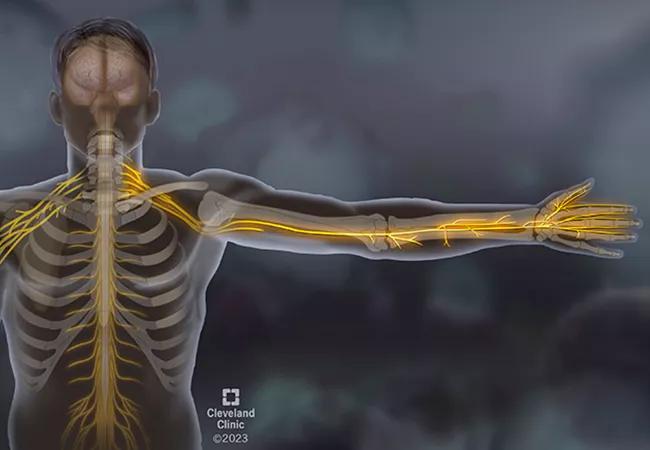
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9adc4f63-c0e7-4208-af42-97e03a75b079/23-RHE-3660360_Sjogrens_Syndrome_CQD-650x450-1-jpg)
Medical illustration
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
A great example of the multidisciplinary aspect of rheumatology is a case recently presented at our department’s Friday morning case conference. For this case, the neurology team joined us during the morning conference to help guide us through their thought processes and teach during the presentation.
A man in his mid-30s with no significant medical history presented to an outside emergency room with bilateral hand numbness that had begun five weeks earlier. At first, the patient thought the hand numbness was carpal tunnel syndrome from using heavy tools in his construction job. The numbness persisted, spreading to the left side of his face and then to the front of his thighs. He also felt weakness in his hands and was having trouble gripping tools.
The progression of symptoms prompted him to be evaluated in the ER, where he was noted to have sensory loss to all the areas he complained about as well as bilateral grip weakness.
In the ER, an MRI of his brain and spine was performed and read as normal. He was transferred to Cleveland Clinic and evaluated by Neurology.
Once evaluated by Neurology on our main campus, it was noted, the patient had sensation loss to his hands, on the left side of his face in V2 and V3 distribution, and in a large patch on the anterior thighs. Importantly, the patient also had significant proprioceptive loss in his fingers and wrists. When he closed his eyes, his arms demonstrated pseudoathetoid movement; they drifted aimlessly. The patient couldn’t tell where his arms were in space without visual input. The patient also experienced ataxia with walking. It was previously noted that the patient had weakness in his hands, but when he was asked to look directly at one of his hands and then grip the examiner’s hand, motor strength was normal.
Advertisement
First, let’s consider non-length-dependent versus length-dependent sensory neuropathy. This patient’s sensory loss followed a patchy distribution, starting in his hands, traveling to half his face, then reaching his thighs. The areas of sensory loss were not dependent on the length of the nerve. In contrast, most neuropathies are length-dependent, meaning the sensory loss starts at the farthest nerves of the feet, such as that seen with diabetic neuropathy.
Next: Consider large-fiber versus small-fiber sensory neuropathy. There are multiple sizes of sensory fibers, each responsible for different aspects of sensation, but we can simplify it to large- and small-fiber sensory nerves. Small-fiber sensory nerves detect pain and temperature. When they’re involved, it’s typically a painful neuropathy. Large-fiber sensory nerves, in contrast, are primarily responsible for proprioception and detecting vibration. In this case, the patient demonstrated very poor proprioception — indeed, so severe that he couldn’t tell where his arms and feet were in space, which explains his pseudoathetoid movements and ataxia.
Upon initial clinical examination, his loss of sensation in the hands made it seem that he was weak. This illusion was overcome by asking the patient to look at his hand while squeezing. Because of the loss of sensation and proprioception, he couldn’t feel the examiner’s hands to squeeze, but once he looked at his hands to squeeze, he was able to perform normally. Now we know he has a primary large-fiber sensory neuropathy with intact motor function.
Advertisement
He does not have any upper motor neuron signs, such as hyperreflexia or spasticity. His brain MRI and spine were normal.
So where are the lesions?
Clinical evidence points to primary large-fiber, sensory non-length-dependent neuropathy. This is consistent with the destruction of the dorsal root ganglion, the bean-shaped bundle of sensory nerves lying adjacent to the spinal cord at multiple levels of the cord. This phenomenon is called a sensory ganglionopathy, and the differential is rather limited.
This is how Rheumatology gets on board.
The differential of a sensory ganglionopathy is limited to malignancy, vitamin toxicity, chemotherapy toxicity, celiac disease or Sjögren’s syndrome. During the workup of this patient, he was found to be SSA-positive. Rheumatology was consulted, and we learned that the patient had been experiencing a multiple-month history of dry eyes and dry mouth preceding his neuropathy symptoms but didn’t think much of it. He was evaluated by Ophthalmology. Objective measures demonstrated eye dryness.
MRI of the spine is typically normal, so the diagnosis is made clinically. The treatment is not standardized, but most experts agree with aggressive immunosuppression upfront in hopes of stopping inflammation and further damage. Pulse dose regimens of methylprednisolone, rituximab, IVIG and plasmapheresis have all been used.
Rheumatologists encounter a variety of pathologies, and interdisciplinary care is a critical component of care. In our Friday morning presentation of this case, a Neurology team was present to guide us through the neurologic findings and explain how the exam changed the differential and workup, narrowing the diagnosis. A rapidly progressive neurologic presentation was worked up and found to be secondary to a primary systemic autoimmune disease. Working with our Neurology colleagues, we developed and initiated an immunosuppression regimen we hope will halt the progression of sensory loss for the patient.
Advertisement
Advertisement
New clinic meets Hispanic patients where they are
Fellows will train under faculty with broad range of expertise
When to suspect concurrent giant cell arteritis
New research explores the role of immune cell and blood-brain barrier changes
Significant advances had a direct impact on clinical practice
Adam Brown, MD, shares his passion for solving rheumatologic mysteries
Distinct MRI signature includes lesions beyond the corpus callosum, features predictive of vision and hearing loss
Benefits of neoadjuvant immunotherapy reflect emerging standard of care