FDA-designated breakthrough device offers first treatment option for severe TR and right HF
In February 2022, Cleveland Clinic interventional cardiologists performed the first implantation of the TricValve® Transcatheter Bicaval Valves System in a patient in North America. The successful case, completed in an 82-year-old man with severe symptomatic tricuspid regurgitation (TR) and right heart failure (RHF), was carried out under a compassionate-use clearance from the FDA.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The TricValve system gained breakthrough device designation by the FDA in December 2020. It consists of two self-expanding biological valves that are implanted percutaneously into the superior vena cava (SVC) and inferior vena cava (IVC) without disturbing the native tricuspid valve. The caval system is intended for patients with severe symptomatic TR and RHF who are deemed to be at extreme/high surgical risk or noncandidates for open surgical therapy.
“TricValve can potentially provide an effective and low-risk solution for many patients who currently have no treatment options,” says Rishi Puri, MD, PhD, a Cleveland Clinic interventional cardiologist who performed the first U.S. case along with Samir Kapadia, MD, Chair of Cardiovascular Medicine at Cleveland Clinic.
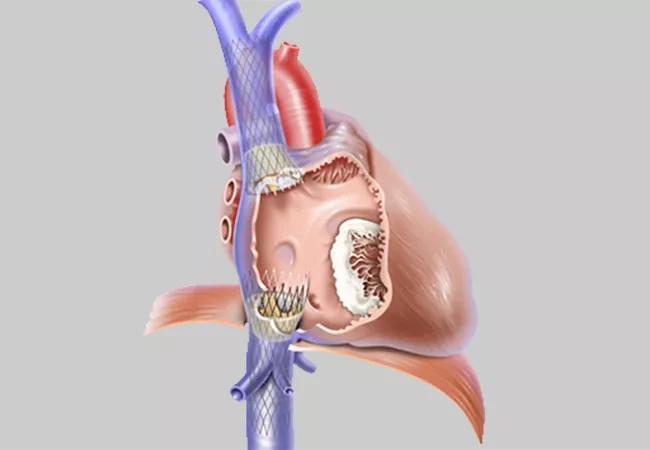
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/adb60982-9d85-4e48-a298-652c308f6001/22-HVI-2843464_transcatheter-bicaval-valves-system_650x450_jpg)
Illustration showing positioning of the two TricValve bioprostheses in the inferior and superior vena cava. Image courtesy of P + F Products + Features GmbH.
Severe TR can be treated with tricuspid valve surgery, but the operation can be too risky for many patients who need it. And medical therapy, which involves increasing dosages of diuretics, is soon “maxed out” for patients with failing kidneys.
In the last several years, several percutaneous device solutions — edge-to-edge repair, direct annuloplasty and orthotopic or heterotopic valve replacement — have been developed to replace or repair the tricuspid valve. However, they are unsuitable for most patients with severe TR due to anatomic issues, the presence of pacemaker leads or periprocedural imaging challenges.
Advertisement
According to Dr. Puri, TricValve shows potential for use in a broad population, even for patients who present late in the disease course and have difficult tricuspid valve anatomy, as caval anatomy is less complex compared with that of the right heart and tricuspid valve.
The two fully pre-mounted devices are implanted via femoral access with fluoroscopy guidance under monitored (light) anesthesia care. The nitinol stents contain bovine pericardium leaflets, with a long skirt on the SVC valve designed to minimize perivalvular leak and a short skirt on the IVC valve to prevent hepatic vein flow occlusion. Several sizes are available, with the choice based on IVC and SVC measurements from a preprocedural CT scan.
The procedure is hemodynamically stable, involving slow controlled release of the device, which is recapturable at up to 80% deployment. Time for implant is typically 30 to 45 minutes, similar to that for self-expanding stents.
“Implantation involves a shallow learning curve for anyone who currently performs transcatheter aortic valve replacement, because the workflow is similar,” Dr. Puri notes.
Device placement prevents regurgitant flow in the IVC and SVC, reducing liver congestion, increasing right ventricle stroke volume into the pulmonary system and improving cardiac output.
TricValve implantation does not touch the native valve and allows for all future tricuspid valve surgical and procedural options, including transcatheter edge-to-edge repair, transcatheter tricuspid valve repair and transseptal puncture, as well as pacemaker implantation.
Advertisement
Two other dedicated caval valve implantation systems are currently under investigation — Tricento and Trillium™ — but have currently undergone less clinical investigation than TricValve.
For a more detailed summary of caval valve implantation, see a recent review article published by Dr. Puri and European colleagues in the Journal of Clinical Medicine (2021 Oct 7;10[19]:4601).
To date, the TricValve system has undergone two single-arm clinical trials in Europe: the TRICUS first-in-human study, involving nine patients in Lithuania, and the TRICUS-EURO study, conducted in 35 patients in Spain and Austria, which led to CE mark approval in May 2021. In addition, more than 200 patients in Europe, Asia and South America have been followed who received implantation through special access or compassionate-use programs.
Procedural success has been high, Dr. Puri notes, with good safety and improved New York Heart Association class and other quality-of-life measures.
To fulfill FDA requirements for TricValve approval in the U.S., a randomized controlled trial called TRICAV will soon be launched under the coordination of the Cleveland Clinic Coordinating Center for Clinical Research (C5Research), with Drs. Puri and Kapadia as its principal investigators. TRICAV is expected to randomize more than 200 patients with severe TR and RHF on a 1:1 basis to receive either TricValve or guideline-directed medical therapy. Some 30 to 40 sites will be included in North America, Europe, Australia and New Zealand. Multiple clinical, functional and imaging endpoints will be evaluated.
Advertisement
“We are eager to begin this study,” says Dr. Puri. “Preliminary evidence indicates that this system is easily implanted and safe, and that it can dramatically improve quality of life for patients who desperately need a therapy option.”
“All current percutaneous techniques are targeting patients with severe symptomatic TR,” adds Dr. Kapadia. “When these patients are screened for current investigational devices to treat TR, less than half meet criteria for these experimental procedures. TricValve will provide a viable treatment option for many of these patients who have no other choices.”
Advertisement
Advertisement

Introducing Krishna Aragam, MD, head of new integrated clinical and research programs in cardiovascular genomics

How Cleveland Clinic is using and testing TMVR systems and approaches

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients

Multimodal evaluations reveal more anatomic details to inform treatment