Even very high-risk patients benefit, and implantation can safely be combined with ablation or TAVR
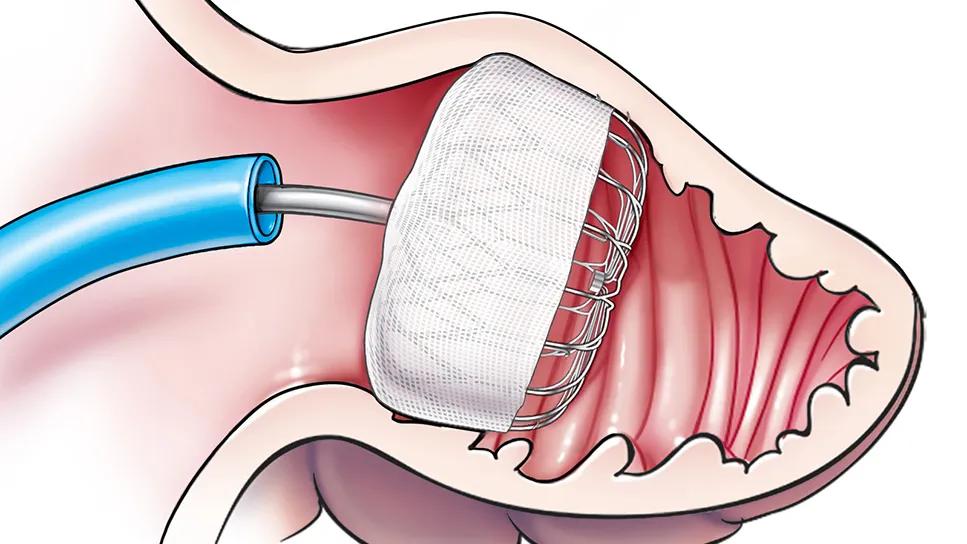
Percutaneous placement of a left atrial appendage closure (LAAC) device offers a safe and effective option for stroke prevention for patients with nonvalvular atrial fibrillation (AF) — even those at very high risk of stroke and bleeding. In addition, select patients can benefit from concomitant LAAC device placement and AF ablation rather than undergoing the procedures separately.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
These are among the conclusions reached by Cleveland Clinic clinicians following implantation of more than 1,000 WatchmanTM LAAC devices at Cleveland Clinic’s main campus, making it the first healthcare institution to surpass this volume milestone. This large number has allowed investigation into the use of the device in special settings.
“We have found the LAAC device to be effective even for our patients at very high risk of stroke,” says Oussama Wazni, MD, Section Head of Cardiac Electrophysiology and Pacing at Cleveland Clinic. “And we are increasingly combining LAAC device implantation with ablation, with excellent results.”
The Watchman device was FDA-approved in March 2015 for treatment of nonvalvular AF in patients needing a nonpharmacologic alternative to warfarin. The parachute-shaped device, delivered percutaneously via the femoral vein, is designed to mechanically block the opening between the atrial appendage and the left atrium, preventing clots formed in the appendage from entering the atrium.
Although use of an LAAC device has been shown in many studies to be a safe alternative to anticoagulation, little is known about its role in patients at very high risk for stroke. From a prospective registry of all patients who underwent Watchman implantation at Cleveland Clinic, 104 consecutive patients with a CHA2DS2-VASc score of 5.0 or more were analyzed. Mean age was 78.5 years, mean CHA2DS2-VASc score was 5.7, and mean HAS-BLED score was 4.0.
At one-year follow-up, ischemic stroke risk was 2.8% (3 patients), which was substantially lower than the estimated risk of stroke based on data from similar patients from previous protocols: 12% for those off anticoagulation and > 4% for patients on warfarin. The study was published in Heart Rhythm (2020;17:27-32).
Advertisement
“We commonly encounter patients who need AF ablation but are not good candidates for long-term anticoagulation, or vice versa,” says Mohamed Kanj, MD, Co-Director of Electrophysiology Laboratories at Cleveland Clinic. “For these patients, we consider placing a LAAC device at the same time as the ablation, as the two procedures share many similar procedural steps.”
Potential advantages of concomitant procedures include reduced procedural risks associated with one versus two procedures, shorter anticoagulation duration and the possibility of lower healthcare procedural costs.
Dr. Kanj adds that other potential candidates for the combined procedures include patients who need a LAAC device and who would benefit from a nonpharmacological rhythm control strategy.
The Cleveland Clinic experience with the concomitant procedures has involved 270 patients to date, with excellent safety and efficacy. The procedures were able to be performed concomitantly in more than 95% of cases.
Dr. Kanj notes that further study will help define the optimal combination of LAAC device implantation and AF ablation.
Another learning from Cleveland Clinic’s 1,000-plus LAAC procedures is the importance of multidisciplinary collaboration. The electrophysiology group works closely with neurologists, gastroenterologists and hematologists so that everyone is on board to optimize the anticoagulation therapy plan. They have developed protocols using up-to-date data on new oral anticoagulants, notes Walid Saliba, MD, Director of Cleveland Clinic’s Atrial Fibrillation Center.
Advertisement
“Even many patients with a history of intracranial hemorrhage can safely take a blood thinner after a certain period of time,” he says. “In some patients we recommend anticoagulants for up to three months after the procedure and then aspirin alone, without the use of clopidogrel.”
The ongoing OPTION clinical trial, for which Dr. Wazni is principal investigator and Cleveland Clinic is a participating site, is designed to determine whether Watchman FLX device implantation is a reasonable alternative to oral anticoagulation following catheter ablation for patients with nonvalvular AF.
The trial consists of 1,600 patients at 130 sites worldwide. Participants must have a CHA2DS2-VASc score of at least 2 for men and at least 3 for women. Treatment arms consist of the following:
Follow-up will be for three years for all-cause death, stroke and systemic embolism as well as nonprocedural bleeding. Trial completion is expected in late 2024.
“Because of the number of participating sites, the OPTION trial will provide real-world evidence on safety and efficacy as these therapies are currently practiced,” says Dr. Saliba, a study co-investigator. “We expect it to support guidance on optimal procedural strategies in this growing population of patients with atrial fibrillation.”
Additionally, data will be available soon from the WATCH-TAVR trial, an investigator-initiated study of the safety and efficacy of performing LAAC at the same time as transcatheter aortic valve replacement (TAVR). This multicenter randomized trial was designed by Samir Kapadia, MD, Chair of Cardiovascular Medicine at Cleveland Clinic, and organized by the Cleveland Clinic Coordinating Center for Clinical Research (C5Research). Enrollment is completed, with 349 patients enrolled and Cleveland Clinic among the top-enrolling sites. The study, now in its follow-up phase, will report results after 24 months of follow-up of the last patient, who was enrolled in November 2020.
Advertisement
“Atrial fibrillation is very common in the TAVR population, and these patients are at high risk of stroke and bleeding,” says Dr. Kapadia. “Left atrial appendage closure is the ideal solution for these patients. When possible, doing TAVR and the Watchman procedure at the same time promises to help reduce stroke and bleeding in these patients.”
Dr. Wazni adds that these proliferating studies of LAAC in additional settings only heighten the significance of the procedure’s burgeoning use. “Our 1,000-case experience demonstrates that LAA closure with Watchman reduces stroke risk in high-risk patients,” he says. “But it also shows this procedure can be combined with TAVR and AF ablation to decrease the risk associated with multiple procedures in addition to lowering risks of stroke and bleeding over the long term.”
Advertisement
Advertisement

How Cleveland Clinic is using and testing TMVR systems and approaches

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients

Multimodal evaluations reveal more anatomic details to inform treatment

Insights on ex vivo lung perfusion, dual-organ transplant, cardiac comorbidities and more