How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR
Cleveland Clinic cardiologists have been deeply involved with mitral valve transcatheter edge-to-edge repair (M-TEER) since the first M-TEER device, MitraClip™, was approved by the FDA in 2013. They took part in clinical trials that confirmed the durability and safety of that device in patients at high risk from open surgery. They developed a method of measuring risk to determine eligibility for M-TEER (Am J Cardiol. 2023;207:39-47) and a method of measuring pressures inside the heart during the procedure to guide results (Struct Heart. 2024;9[2]:100361). They also participated in a head-to-head trial (JACC Cardiovasc Interv. 2023;16[23]: 2803-2816) comparing MitraClip with the second FDA-approved M-TEER device, PASCAL.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
As M-TEER has become one of the fastest-growing procedures in interventional cardiology, recent efforts have focused on exploring use of M-TEER in new patient populations. In the process, Cleveland Clinic staff have developed M-TEER expertise and shared their knowledge by publishing over 60 papers on various aspects of M-TEER to date.
“The strength of our program lies in the volume of M-TEER cases we do — about 200 annually — and the fact that we’ve been doing them since the inception of M-TEER,” says Amar Krishnaswamy, MD, Section Head of Invasive and Interventional Cardiology.“The procedure relies heavily on guidance by cardiac imagers, and we have imagers dedicated to these procedures who are international pioneers in their own right.”
Recommendations for M-TEER are based on the etiology of a patient’s mitral regurgitation (MR).
MR is considered secondary (or functional) when it is caused by a compromised ventricle or atrium rather than damage to the mitral valve leaflets themselves. In patients with secondary MR, surgical valve repair or replacement has not demonstrated the same safety or efficacy as M-TEER.
“Edge-to-edge repair has gained a strong foothold in the care of these patients and has a higher recommendation in the guidelines than cardiac surgery does,” Dr. Krishnaswamy notes.
M-TEER was approved for secondary MR based on results of the multicenter COAPT trial in 614 patients with heart failure and moderate-to-severe or severe secondary MR randomized to MitraClip therapy or guideline-directed medical therapy. Through five years of follow-up, rates of heart failure hospitalization and death were significantly lower with MitraClip than with medical therapy.
Advertisement
“COAPT showed we can save one life and prevent hospital admissions by treating only six or seven patients with M-TEER,” says Cleveland Clinic Cardiovascular Medicine Chair Samir Kapadia, MD, a COAPT lead investigator and member of the trial’s steering committee.
“Randomized clinical trial data demonstrate both functional and survival benefit with M-TEER in patients with ventricular dysfunction and secondary MR,” adds Dr. Krishnaswamy. “However, we believe that large numbers of these patients are not being referred for edge-to-edge repair. That needs to change.”
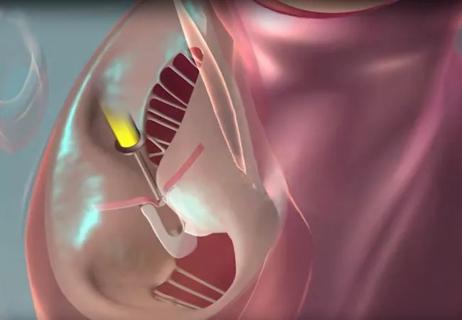
In the United States, the most common cause of primary MR is a flail mitral valve leaflet due to fibroelastic deficiency (Figure). For patients with this presentation, surgical valve repair (or replacement, if necessary) remains the gold-standard treatment.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/05d9ff3a-f23d-4681-afec-dd2704b5f39e/25-HVI-6845760-inset)
However, some of these patients are at high risk from surgery due to advanced age and/or comorbidities, which prompted interest in transcatheter treatment options. Early studies of MitraClip — including the EVEREST II randomized trial and high-risk registry, which formed the basis of the device's FDA approval — revealed that it yielded the greatest benefit in patients at high surgical risk.
That population at high or prohibitive surgical risk has been the domain of M-TEER use for primary MR in subsequent years, with the PASCAL system also gaining FDA approval in 2022. The PASCAL and MitraClip systems were directly compared in this population in the international randomized CLASP IID trial, in which Cleveland Clinic was a participating center. The trial found no significant differences in clinical outcomes, complications or mortality through at least one year, with follow-up ongoing.
Advertisement
Cleveland Clinic uses both FDA-approved M-TEER devices for patients with primary MR. “We believe that each device may be better suited to certain patient anatomies, so having a choice is helpful,” says Dr. Krishnaswamy.
Meanwhile, Cleveland Clinic plays a leading role in the National Institutes of Health-sponsored PRIMARY trial (NCT05051033) randomizing patients with primary MR to surgical mitral valve repair or M-TEER with an approved device. The trial is notable because it includes patients at all levels of surgical risk, with those at low risk representing most enrollees to date. (The PRIMARY trial is profiled in detail in a companion Consult QD article.)
“I expect that M-TEER’s indications for primary MR will broaden over time to include patients at different levels of surgical risk, but we’ll need to wait a few years for the data,” Dr. Krishnaswamy says. “Notably, however, there are a number of patients with primary MR who come to us for M-TEER whose anatomy we determine to not be suitable for it. Surgery will always have an important role because it has such good efficacy and safety in primary MR.”
The durability of M-TEER is not yet known, either, since long-term data are not available. Other issues have come to light that also require further investigation. “There are data suggesting that up to 50% of valves may need to be repaired, even after clipping, and that the PASCAL clip may preclude future surgical repair of the valve,” Dr. Kapadia says. “That’s why if a patient is a good surgical candidate, we still recommend surgery.”
Advertisement
At Cleveland Clinic, decisions between surgical mitral valve repair, M-TEER or any other treatment options are made by a heart team comprising an interventional cardiologist, a cardiac surgeon, an imaging cardiologist, a heart failure cardiologist and sometimes an electrophysiologist. A coordinator orders the necessary imaging tests, which are performed by cardiovascular imaging specialists highly versed in advanced imaging software and probes. The team meets once a week to review the tests and recommend the best approach for each patient. All outcomes are tracked.
“The partnership with our cardiac imaging colleagues and recent advances in procedural echocardiography have broadened the scope of patients we can treat and allowed us to treat all patients better,” says Dr. Krishnaswamy.
In Ohio, such teams come together to offer and perform M-TEER at two of Cleveland Clinic’s regional hospitals in addition to its Main Campus. Most patients are discharged the day of the procedure or the day after.
The procedures are performed two days a week in hybrid operating rooms. “In this way, if there is a complication, we can easily convert to open surgery, but that happens in less than one case a year,” Dr. Kapadia notes. “M-TEER is one of the safest procedures we do. Adverse events like bleeding and stroke are extraordinarily rare.”
He continues: “If you look at the average experience, 12 M-TEER procedures a year is generally considered a reasonable frequency for an operator. We do 200 a year. That builds levels of efficiency, safety and procedural efficacy among our teams that are difficult to match.”
Advertisement
Advertisement

How Cleveland Clinic is using and testing TMVR systems and approaches
And substudy reveals good outcomes with PASCAL system in patients with complex mitral valve anatomy

Scenarios where experience-based management nuance can matter most

NIH-funded comparative trial will complete enrollment soon

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients

Multimodal evaluations reveal more anatomic details to inform treatment