Second case ever reported highlights complexity, rarity
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
A 60-year-old male presented with a six-year history of intermittent daily fever as high as 103.5 F lasting three to four weeks. Associated symptoms included erythematous rash in his torso, peritoneal symptoms, myalgias, arthralgias, bilateral episcleritis and lymphadenopathy. Neither prednisone nor colchicine proved effective. Extensive work up for fever of unknown origin was inconclusive. His symptoms were reminiscent of tumor necrosis factor receptor (TNFR)-associated periodic syndrome (TRAPS), but TRAPS would be unusual to encounter in late adulthood.
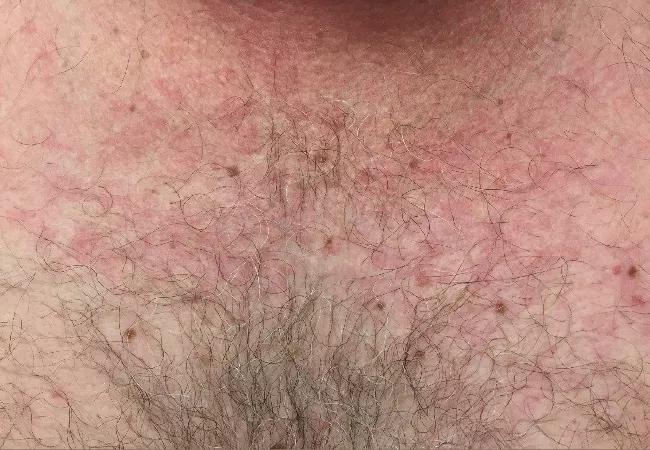
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/390e9c5c-a5fb-410f-9691-f9c79e88d8a2/17-RHE-1243-Kontzias-Image-02-650pxl-width_jpg)
Erythematous rash on the patient’s torso.
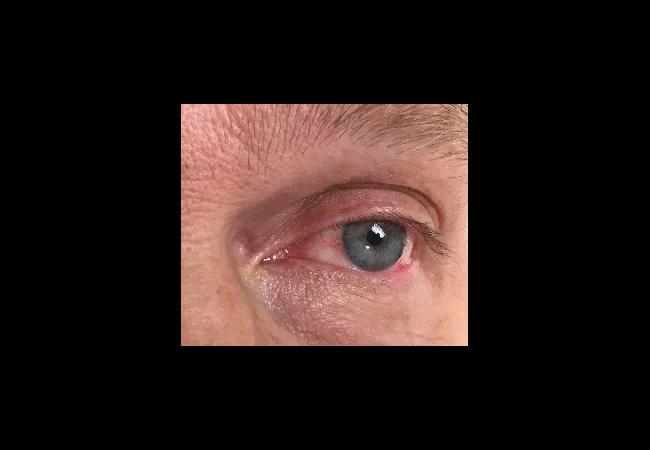
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/e8c9e056-3871-4e44-844e-06262d5811a1/17-RHE-1243-Kontzias-Image-01-650pxl-width_jpg)
Bilateral episcleritis associated with intermittent daily fever.
TRAPS is a rare, autoinflammatory, autosomal-dominant disease caused by gain-of-function mutations in the TNFRSF1A gene, which encodes the 55-kd TNFR type I (TNFRI) protein. Its estimated prevalence is one per million individuals. Typically, autoinflammatory diseases lack serologic evidence of adaptive immunity involvement such as high-titer auto-antibodies. Nonspecific laboratory evidence of systemic inflammation — high erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), leukocytosis, thrombocytosis — is typical during flares.
Although AIDs usually present in childhood or early adulthood, they are increasingly recognized across age groups with the help of next generation sequencing (NGS). Many of the prototypic autoinflammatory disorders are caused by mutations in single genes that encode proteins involved in innate immunity pathways. In the genomics era, NGS has become accessible and employed in the rheumatology clinic as a tool to dissect clinical phenotypes that belong to the spectrum of AID. NGS has the capacity to detect any de novo sequence variants beyond what can be represented on single-nucleotide polymorphism arrays. NGS can also detect even low-level mosaicism, the presence of two or more populations of cells with different genotypes in one individual.
Advertisement
To diagnose this patient, we worked with the Center for Personalized Genetic Healthcare and the Department of Molecular Pathology. Using NGS, we found evidence of somatic mosaicism on the F89L variant, which has been previously associated with TRAPS. The mutant allele’s presence in buccal mucosa and whole blood, and absence in hair root supported somatic TNFRSF1A mosaicism. In silico prediction modeling with SIFT and Polymorphism Phenotyping-2 prediction modeling showed numerous structural rearrangements from this mutation, resulting in changes in the protein surface profile.
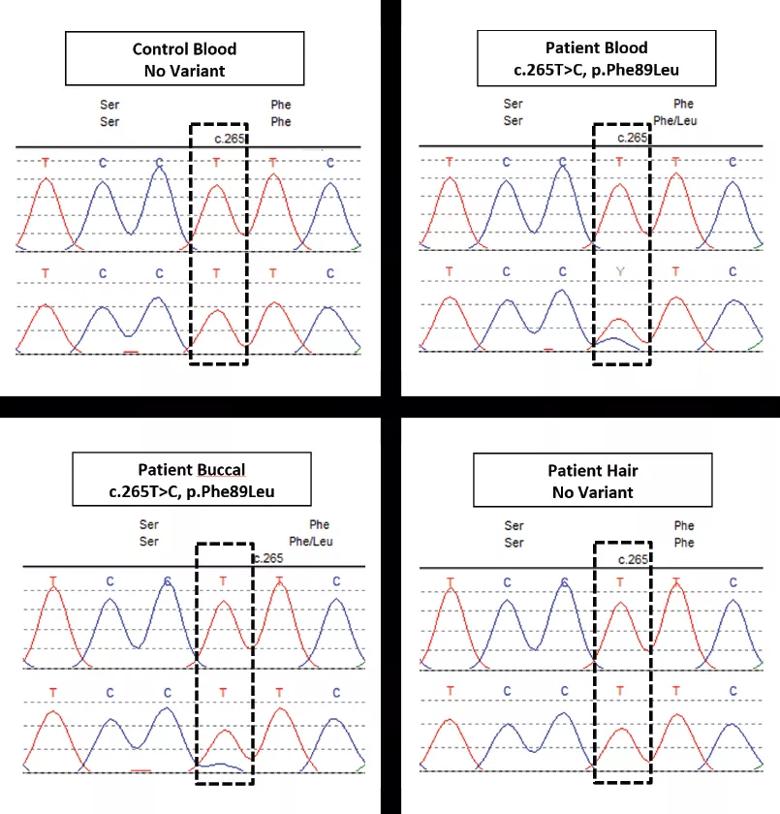
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/f2078ea7-0cb0-4db3-a4d8-ba43cc5cd78c/17-RHE-1243-Kontzias-Image-03-805pxl-width_jpg)
The variant present in the patient’s blood (B) versus the control (A) is also present in the buccal sample (C) but not in the hair sample (D).]
Identifying this variant through NGS allowed us to confirm a suspected diagnosis and provide targeted treatment with canakinumab. With treatment, the patient’s symptoms resolved and protein levels normalized. The complete response to an interleukin-1 blocker further supported the TRAPS diagnosis.
Since autoinflammatory disorders were first described nearly 20 years ago, the causes of these confounding syndromes have at times remained a mystery. Recent advances like NGS have brought welcome news for clinicians and patients, revealing important clues about the genetically mediated aspects of these diseases. Both this and the only other case ever reported suggest that postzygotic mutations may cause TRAPS phenocopies in adulthood, offering yet another clue into the genesis of autoinflammatory disorders. I presented this case with Cassandra Calabrese, DO, a fellow in the Department of Rheumatology, and Yu-Wei Cheng, PhD, staff in the Genomics Institute, at the Annual European Congress of Rheumatology in 2017, and we are conducting further studies to identify the extent of mosaicism in this patient’s cell subsets.
Advertisement
Our Clinic for Adult Autoinflammatory Diseases is one of the very few multidisciplinary clinics across the country focusing on investigating and managing these enigmatic diseases. We believe strongly that multidisciplinary care of autoinflammatory conditions yields the best outcomes and is imperative for these challenging cases for proper diagnosis, treatment and genetic counseling.
Dr. Kontzias directs the Clinic for Adult Autoinflammatory Diseases in the Department of Rheumatic and Immunologic Diseases.
Advertisement
Advertisement

The case for continued vigilance, counseling and antivirals

High fevers, diffuse rashes pointed to an unexpected diagnosis

No-cost learning and CME credit are part of this webcast series

Summit broadens understanding of new therapies and disease management

Program empowers users with PsA to take charge of their mental well being

Nitric oxide plays a key role in vascular physiology

CAR T-cell therapy may offer reason for optimism that those with SLE can experience improvement in quality of life.

Unraveling the TNFA receptor 2/dendritic cell axis