The benefits of using this methodology and results from a single-institution experience
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Autosomal recessive polycystic kidney disease (ARPKD) is a potentially lethal genetic disorder that affects approximately 1 in 20,000 children. ARPKD shares common cellular pathophysiologic features with other cystic kidney diseases but is clinically and genetically distinct from other “ciliopathies,” including autosomal dominant PKD (ADPKD). ARPKD is characterized by diffuse fusiform dilatations of the collecting tubules, which often leads to the classic appearance of enlarged echogenic kidneys on ultrasonography.
Morbidity and mortality are significant: 30% of ARPKD children die in the neonatal period and 40% of survivors progress to end-stage renal disease (ESRD) by age 15. There are currently no disease-specific, clinically available therapies for ARPKD, and treatment is directed at the management of hypertension (often severe) and chronic kidney disease (CKD) complications.
Several novel therapies have shown promise in ARPKD animal models. Unfortunately, a major roadblock for implementing clinical trials in affected patients is the absence of sensitive measures of ARPKD kidney disease progression, which could be used to identify “high risk” patients and monitor response to therapies during a clinical trial.
Current clinical endpoints of CKD progression, such as doubling of serum creatinine or greater than 30% reduction in estimated glomerular filtration rate (eGFR) over a two- to three-year period, have substantial limitations in assessing ARPKD progression. Based on data we previously analyzed from the Chronic Kidney Disease in Children (CKiD) study, it is estimated that more than 400 ARPKD subjects would be required for a four-year clinical trial in order to detect a significant change in the rate of GFR decline, a prohibitive number for this rare disease. These findings highlight the need for more sensitive markers to measure kidney disease progression.
Advertisement
Non-invasive magnetic resonance imaging (MRI) techniques hold promise as surrogate endpoints for ARPKD progression. Unfortunately, conventional MRI-based volumetric measures, such as those used successfully to measure progression and response to therapy in ADPKD, are not applicable to ARPKD, as kidney size stabilizes or decreases over time despite worsening cystic disease. To address this important clinical need, our multidisciplinary team has developed and applied novel quantitative MRI techniques to the study of ARPKD.
In previous animal studies in two different ARPKD rodent models (the PCK rat and the bpk mouse), we identified T1 and T2 mapping as potential biomarkers of kidney disease, with strong correlations to histologic measures of disease.
We have subsequently applied these techniques to the study of human ARPKD kidney disease. In these investigations, we have utilized a novel MRI methodology, called magnetic resonance fingerprinting (MRF), which was initially pioneered at our university. MRF is a unique quantitative MRI acquisition and image processing methodology that provides rapid, simultaneous and accurate measures of multiple MRI tissue properties (e.g. T1, T2, perfusion) with reduced motion artifacts (and no requirement for IV contrast agents) in comparison to conventional quantitative MRI techniques. The resistance-to-motion artifact is particularly crucial in abdominal imaging in younger children, for whom respiratory and other motion artifacts can significantly limit acquisition of accurate MRI data. Importantly, the application of MRF to the study of ARPKD kidney disease has the potential to eliminate the need for sedation in these young children, which would be associated with increased risk and costs when utilized in clinical trials.
Advertisement
Our group recently developed a new MRF methodology that acquires T1 and T2 maps in 15 seconds / imaging slice on a human 3.0T MRI scanner. After performing validation in healthy volunteers (N=10), we evaluated this new MRF methodology in ARPKD subjects with mild-moderate CKD (N=7).
As shown in Figure 1, kidney T1 and T2 maps from a healthy volunteer (left column) as well as two pediatric patients with ARPKD (middle and right columns) illustrate the ability of MRF to detect renal cystic burden. Specifically, the T1 and T2 maps visibly distinguish the ARPKD child with more severe disease from one with mild disease and both are clearly distinct from the healthy volunteer.
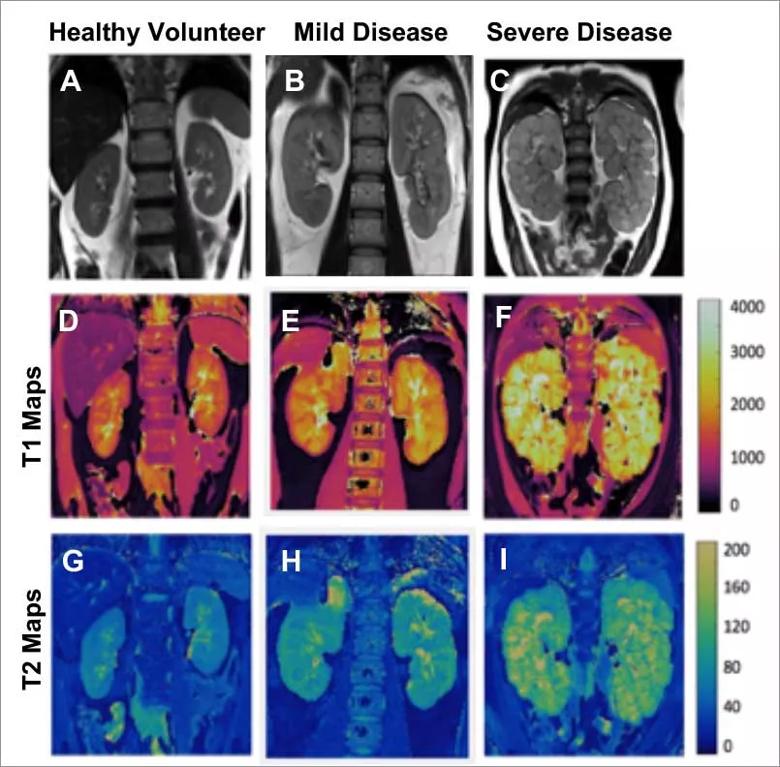
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/be6c9e73-5626-413a-9425-ce41934a70bc/Inset-1-1_jpg)
Figure 1. MRF in ARPKD Kidney Disease A-C Representative conventional anatomic MRI images; D-F MRF-based T1 maps; and G-I MRF-based T2 maps for a healthy volunteer (left column), an ARPKD patient with mild kidney disease (middle column), and an ARPKD patient with more severe disease (right column). Note the increased kidney T1 and T2 values (visibly brighter appearance on the corresponding maps) that reflect increasing cystic burden in the ARPKD patients.
To assess repeatability, an essential aspect of imaging studies, ARPKD subjects underwent imaging on two sequential days (Figure 2).The MRF- derived T1 and T2 maps both showed excellent repeatability: the mean difference in T1 and T2 values between successive days for the groups as a whole was only 1.9% and 2.1%, respectively. Finally, in order to further interrogate the ability of this novel MRF technique to accurately distinguish cystic kidney disease across a spectrum of ARPKD disease, we then examined the relationship between MRF-based mean T1 and T2 and renal function (eGFR) in the cohort.
Advertisement
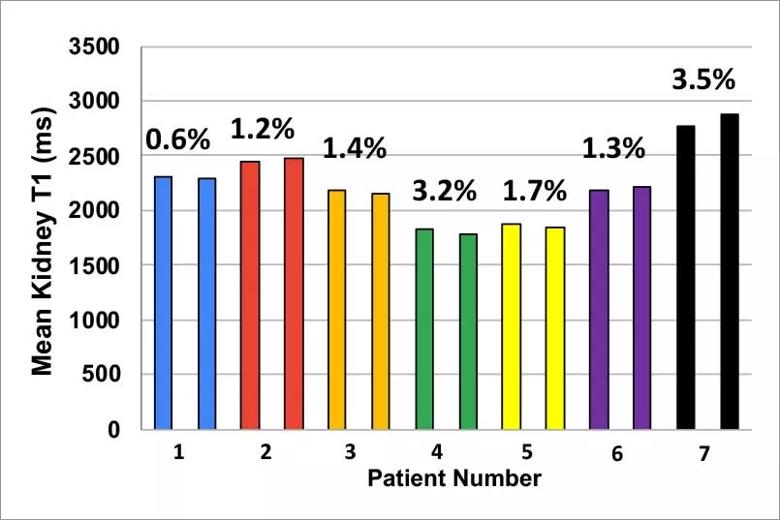
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/dedd18da-55af-4207-9776-52a6d8a36558/Inset-2a_jpg)
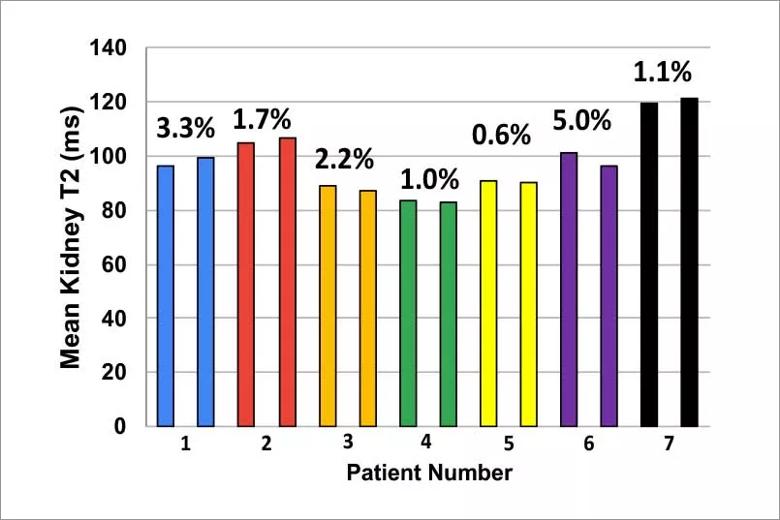
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/626def32-1aee-4c98-910f-36a1f98cd7bc/Inset-2b_jpg)
Figure 2. Repeatability of MRF-derived kidney T1 and T2 values in ARPKD subjects. Repeat scans on seven ARPKD patients obtained on two successive days. Individual patients are color-coded (first bar =Day 1; second bar= Day 2). Note the vey low variance in the repeat kidney T1 and T2 values for each subject across the spectrum of disease (low T1/T2 values= milder disease, higher T1/T2 = more severe disease).
As shown in Figure 3, mean T2 showed a significant correlation with eGFR. Mean T1 also showed a correlation but did not reach significance.
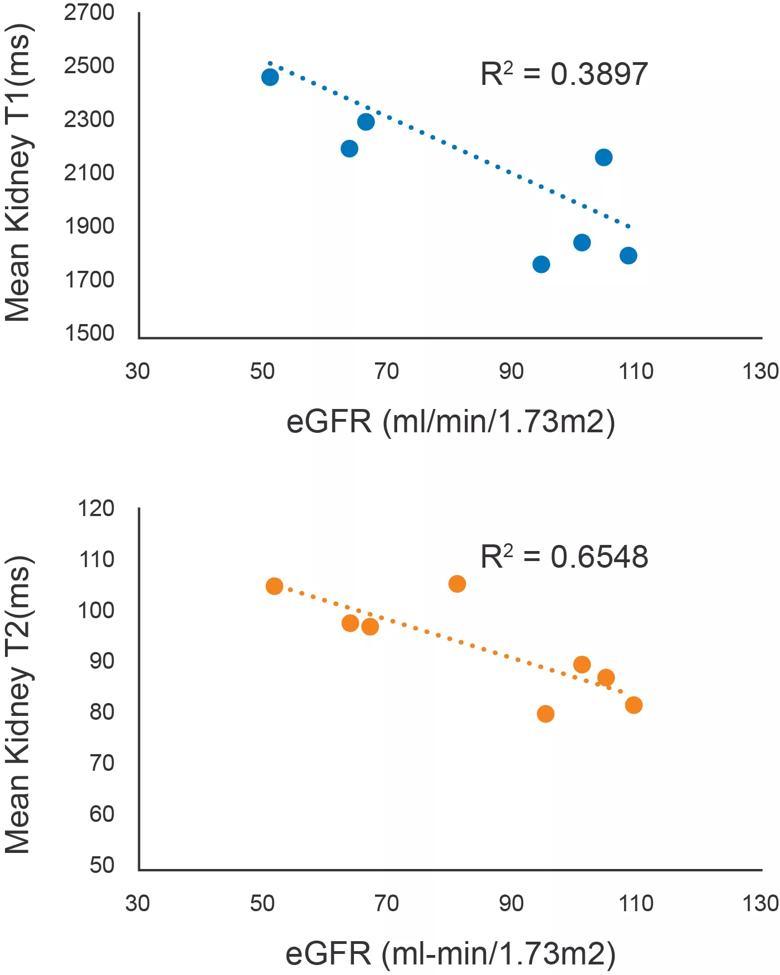
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b9077432-fd61-4727-9356-6e6d087e2da0/21-CHP-2509263-v2-1-2_jpg)
Figure 3. Relationship between MRF-derived T1 and T2 values and Renal function in ARPKD. Mean T1 and T2 values for the ARPKD patients (n=7) are plotted against eGFR (using the recently reported U25 CKID formula). T2 showed a significant negative correlation. T1 also showed a correlation but did not reach significance.
These encouraging, first-ever initial studies of MRF in ARPKD children highlight the potential for MRF-derived mean T1 and T2 to serve as biomarkers or ARPKD kidney disease progression. Additional longitudinal studies are underway to further define this potential. If one or both of these biomarkers are found to accurately assess disease severity, they could be utilized to identify patients at high risk for progression for enrollment in treatment trials, thereby providing ARPKD patients with access to potential life-prolonging therapies.
This research was supported by grants from the NIH/NIDDK and the PKD Foundation
References
Advertisement
Dell KM, Matheson M, Hartung EA, Warady BA, Furth SL, Chronic Kidney Disease in Children S. Kidney Disease Progression in Autosomal Recessive Polycystic Kidney Disease. J Pediatr 2016;171:196-201 e1.
Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature 2013;495:187-92.
MacAskill CJ, Erokwu BO, Markley M, Parsons A, Farr S, Zhang Y, Tran U, Chen Y, Anderson CE, Serai S, Hartung EA, Wessely O, Ma D, Dell KM, Flask CA. Multi-parametric MRI of kidney disease progression for autosomal recessive polycystic kidney disease: mouse model and initial patient results. Pediatr Res 2021;89:157.
MacAskill CJ, Markley M, Farr S, et al. Rapid B1-Insensitive MR Fingerprinting for Quantitative Kidney Imaging. Radiology 2021;300:380-7.
About the author: Dr. Dell is a pediatric nephrologist in the Center for Pediatric Nephrology and Hypertension, Vice Chair of Research for the Pediatric Institute and Professor of Pediatrics, Cleveland Clinic Lerner College of Medicine at Case Western Reserve University.
Advertisement

Findings hold lessons for future pandemics

One pediatric urologist’s quest to improve the status quo

Overcoming barriers to implementing clinical trials

Interim results of RUBY study also indicate improved physical function and quality of life

Innovative hardware and AI algorithms aim to detect cardiovascular decline sooner

The benefits of this emerging surgical technology

Integrated care model reduces length of stay, improves outpatient pain management

A closer look at the impact on procedures and patient outcomes