Larger data set confirms safety, efficacy and durability for SFA lesions over 20 cm
There’s more evidence that the DETOUR™ percutaneous transfemoral arterial bypass (PTAB) system ― which received FDA approval in June 2023 ― is a viable alternative to open surgical bypass for long-segment, complex superficial femoral artery (SFA) disease. Ad hoc analysis of aggregated data from the two prospective, single-arm DETOUR 1 and DETOUR 2 trials, involving 273 patients with two-year follow-up, demonstrates that percutaneous transarterial bypass for complex peripheral artery disease (PAD) lesions is safe and effective, with good durability. Outcomes were presented as a late-breaking clinical trial at the VIVA (Vascular InterVentional Advances) 2023 conference.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“Combined data from the similarly designed international DETOUR trials further support the use of this PTAB method for long, complex SFA lesions,” says presenter Sean Lyden, MD, a study principal investigator and Chair of Vascular Surgery at Cleveland Clinic. “These results are very encouraging that this alternative to traditional open prosthetic bypass surgery will become a more commonly used intervention for patients who currently have endovascular options with limited durability.”
Open femoral-popliteal bypass surgery is the gold standard for treatment of long, complex femoropopliteal lesions. However, its use is limited due to risks of high morbidity, lengthy hospital stays and high readmission rates. Although endovascular intervention is possible for such lesions, restenosis commonly occurs, which limits durability.
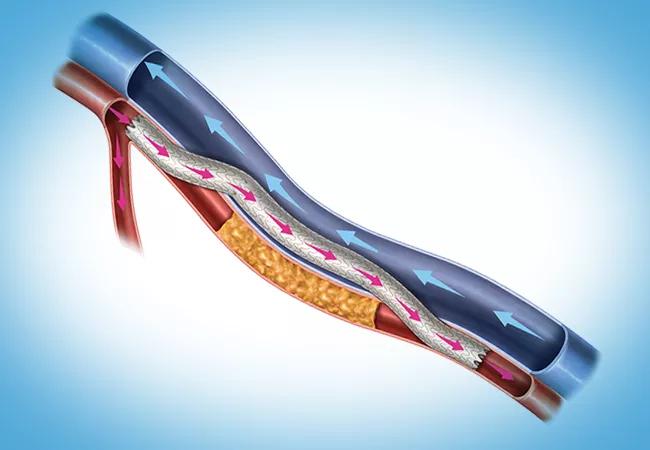
The DETOUR system is designed to be a completely endovascular approach for accomplishing bypass for patients with symptomatic femoropopliteal disease involving long lesions (20-46 cm) with chronic total occlusions, in-stent restenosis or diffuse stenosis (>70%) with moderate to heavy calcification. The system involves construction of a percutaneous femoropopliteal bypass using standard endovascular techniques, the Endocross™ novel crossing device and the TORUS™ stent graft (Figure). During the procedure, the interventionalist enters the SFA origin and crosses into the femoral vein using the Endocross at least 3 cm distal to the origin of the vessel and then travels down the femoral vein and reenters the popliteal artery, landing above the tibial plateau in a nondiseased portion of popliteal artery. TORUS stent grafts are then lined from the distal end to the SFA origin.
Advertisement
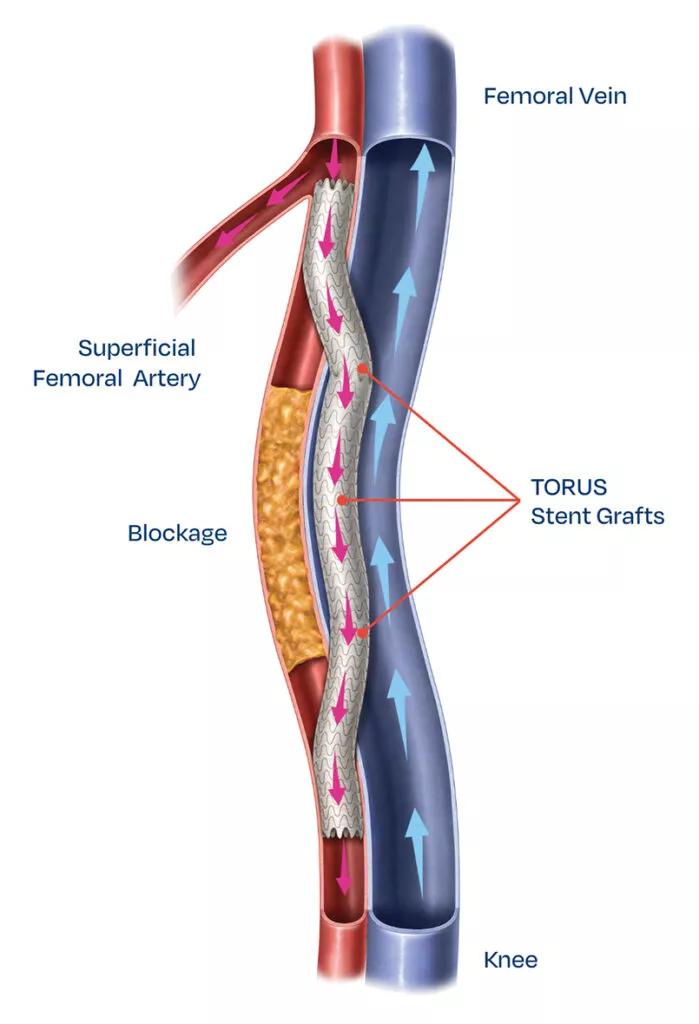
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9d7b9555-b491-4c30-bc29-5cf3fd085a56/23-HVI-4391409-CQD-Inset1-699x1024_jpg)
Figure. Illustration of the femoropopliteal bypass created with the DETOUR system. Image provided courtesy of Endologix LLC.
“The Endocross device has a powerful spring-loaded needle to penetrate the artery and vein,” says J. Eduardo Corso, MD, a colleague of Dr. Lyden’s in Cleveland Clinic’s Department of Vascular Surgery. “This is especially needed at the distal anastomosis and works well for reentry.”
The system has been tested in two similar prospective, single-arm international trials:
Advertisement
A continued access study of DETOUR 2 (NCT04625660) is in progress, with follow-up to three years.
The current aggregate analysis was conducted on variables common to both studies.
The analysis included 273 patients treated with the DETOUR system (mean age of 68 years, 76.3% male). Baseline comorbidities included coronary artery disease (87.6%), hypertension (86.5%), diabetes (44%) and renal insufficiency (9.8%). Mean lesion length was 31.6 cm and included 94% chronic total occlusions and 14% in-stent restenotic lesions. Half the patients had a previous PAD intervention and 16% had a previous PAD surgery.
Procedural outcomes included a mean procedure time of 166 ± 92 minutes and fluoroscopy time of 46 ± 20 minutes. Mean contrast volume was 204 ± 104 mL. Estimated blood loss was 54 ± 60 mL. Mean length of stay was 1.3 days.
DETOUR 1 and DETOUR 2 showed agreement on efficacy endpoints. Specific clinical outcomes included the following:
“PTAB demonstrates high procedural success and two-year efficacy comparable to that of open prosthetic bypass for long, complex SFA lesions,” Dr. Lyden concludes. “Unlike surgery, this intervention does not require general anesthesia and avoids long hospital stays and high risk of complications.”
Advertisement
He adds that third-year DETOUR 2 trial results, along with real-world data from registries over time, are needed to confirm and extend these two-year findings.
Meanwhile, experience with the DETOUR system is mounting since it became commercially available last year. “The patients I have treated with this system have had good short-term results and shorter length of stay compared with surgical bypass,” says Dr. Corso. “It allows some distal above-knee targets to be reached beyond what is easily accessible from a medial surgical approach. In the right patients, this allows preservation of a below-knee distal target with a good result. In the setting of prior SFA stenting with thrombosis or heavy calcification, this system provides a good alternative.”
Advertisement
Advertisement

Early learnings on use of the minimally invasive alternative to surgical bypass

3 specialists share multidisciplinary perspectives on a widely impactful cardiovascular condition

Two cardiac surgeons explain Cleveland Clinic’s philosophy of maximizing arterial graft use
Study finds that in the absence of ACS, the yield is low and outcomes are unaffected
Benefits of new FDA safety determination go beyond expanding patients’ options

Scenarios where experience-based management nuance can matter most

Introducing Krishna Aragam, MD, head of new integrated clinical and research programs in cardiovascular genomics

How Cleveland Clinic is using and testing TMVR systems and approaches