Results from largest randomized trial of the technology to date
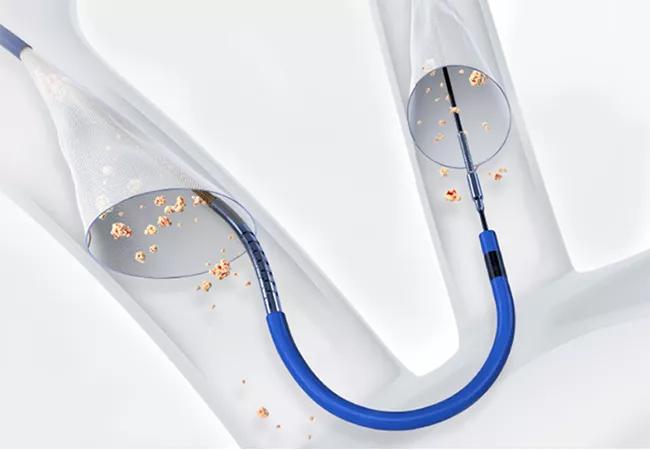
Use of a cerebral embolic protection (CEP) device during transcatheter aortic valve replacement (TAVR) significantly reduced the risk of disabling stroke, although not the overall risk of stroke, in the multicenter PROTECTED TAVR randomized controlled trial.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Results of the study — the largest randomized prospective trial of CEP use in TAVR to date — were presented in a late-breaking clinical science session at the Transcatheter Cardiovascular Therapeutics (TCT) conference by Samir Kapadia, MD, Chair of Cardiovascular Medicine at Cleveland Clinic, and simultaneously published online in the New England Journal of Medicine.
“Given the reduction in disabling stroke demonstrated in this trial, along with the feasibility and safety of using the CEP device, CEP should be considered for all patients undergoing TAVR,” says Dr. Kapadia, global principal investigator of PROTECTED TAVR and first author of the study.
He argues that this recommendation is justified despite the finding that the 21% relative reduction in the study’s primary endpoint — periprocedural stroke — achieved with CEP did not reach statistical significance. He cites the 62% relative reduction in disabling stroke with CEP, noting that this is a critical component of the primary endpoint (although a secondary endpoint) that is dreaded by patients — and therefore highly clinically meaningful. Moreover, the study authors conclude that “while a difference in periprocedural stroke with CEP use was not demonstrated, the results of the study do not exclude a possible benefit.”
“This trial and previous experience have shown the CEP device to be extremely safe,” Dr. Kapadia observes. “In light of that, this evidence of a significant reduction in disabling stroke — not to mention the remaining possibility of a reduction in overall stroke, as suggested by previous single-center and registry data — leaves cost as the only reason not to use CEP. While cost can be an important factor, it is not sufficient to exclude clinical consideration of CEP.”
Advertisement
“It’s important to practice medicine on a foundation of scientific data, and also to place this data in the context of experience and rationale,” adds study co-author Amar Krishnaswamy, MD, Section Head of Interventional Cardiology at Cleveland Clinic. “It is telling that of the expert panel gathered for the PROTECTED TAVR trial presentation at TCT, all but one said they would want CEP used if their family member were undergoing TAVR.”
The rationale for CEP is the embolization of debris from the valve or vasculature that occurs during TAVR, potentially causing periprocedural stroke and resultant morbidity and mortality. Although the emergence of newer-generation TAVR devices has reduced the risk, stroke remains an important complication of TAVR, conferring mortality of approximately 16% at 30 days. “Patients continue to be understandably troubled by the risk of stroke,” Dr. Kapadia notes.
To date, only one CEP device is commercially available in the U.S. — the Sentinel™ Cerebral Protection System, approved by the FDA in 2017. Although the pivotal trial showed the device to be safe and to capture debris in 99% of patients undergoing TAVR, it wasn’t powered to assess the effect on stroke rates. While subsequent nonrandomized single-center studies and registry analyses have shown a significant reduction in stroke rates and mortality with CEP, definitive evidence from a large randomized trial had been lacking prior to PROTECTED TAVR.
PROTECTED TAVR investigators enrolled 3,000 patients at 51 centers in North America, Europe and Australia between February 2020 and January 2022. Patients were randomized to receive TAVR with (n = 1,501) or without (n = 1,499) the Sentinel CEP device. All patients underwent neurological examination before and after the procedure.
Advertisement
The primary endpoint was stroke within 72 hours of TAVR or discharge (whichever was first) in an intention-to-treat analysis. Secondary outcomes were disabling stroke, mortality, transient ischemic attack (TIA), delirium, major vascular complications at the CEP device access site, and acute kidney injury.
The two treatment groups were statistically similar except for a larger share of female patients in the CEP group (42.0% vs. 37.8%).
Major results included the following:
In their study report, the authors identify a few factors that may have contributed to the lack of a significant treatment effect on the study’s primary endpoint of periprocedural stroke:
Advertisement
The authors note that of the eight patients in the CEP arm who had a disabling stroke, two had hemorrhagic strokes, leaving six disabling ischemic strokes — i.e., strokes that could be addressed by CEP. Of these six, one occurred in a patient in whom CEP could not be delivered (the study used intention-to-treat analysis), one in a patient with an embolized valve who also needed resuscitation during the procedure, and one in a patient with stroke-like clinical symptoms but in whom lesion localization was uncertain. Two other strokes occurred in the occipital lobes, which are not fully protected by the studied CEP device. “That leaves just one disabling ischemic stroke observed in the middle cerebral artery territory that the CEP device was designed to protect,” Dr. Kapadia points out.
Additionally, Dr. Krishnaswamy notes, the observed 62% relative risk reduction in disabling stroke — a clinical finding that is easily distinguished by caregivers not trained in neurology — is similar to the 60% to 80% relative risk reduction in all previous single-center studies of CEP, which did not use a trained neurologist in assessing this outcome. “While strictly conjectural,” he says, “this suggests consistency in this finding.”
The authors report that the number needed to treat with CEP to prevent one disabling stroke is 125. “Given patient fears of disabling stroke, this is likely to be deemed important by many patients and caregivers,” says Dr. Kapadia.
It is also deemed important by the TAVR team at Cleveland Clinic, where CEP use is routine for all TAVR patients. Dr. Kapadia says that practice will not change in the wake of this study. “CEP makes sense mechanistically, it is exceedingly safe, and it reduces disabling stroke and may still be shown to significantly reduce stroke overall,” he says. “I would advise all TAVR teams to seriously consider it.”
Advertisement
“We always discuss the risks of TAVR and surgical AVR frankly with our patients before either procedure,” notes Cleveland Clinic cardiac surgeon James Yun, MD, PhD. “Frequently, patients and family members are relieved to hear that we have a CEP device for use with TAVR that has any potential to mitigate stroke risk.”
The next big development on this question will be results from the British Heart Foundation’s ongoing BHF PROTECT-TAVI randomized trial of the Sentinel device in nearly 8,000 patients. After that, a patient-level prospective meta-analysis of the combined PROTECTED TAVR and BHF PROTECT-TAVI data is planned.
The PROTECTED TAVR trial was funded by Boston Scientific, which markets the Sentinel device. Dr. Kapadia reports that he has not been compensated by Boston Scientific for his work on the trial.
Image at top shows the Sentinel device. ©2022 Boston Scientific Corporation or its affiliates. All rights reserved.
Advertisement

Aim is for use with clinician oversight to make screening safer and more efficient

Increasing treatment options are extending the window for continued functional gains
Study links large artery atherosclerosis and delayed presentation to poorer treatment results

$3.2 million grant will fund use of calcium-based imaging to record neuronal activity in ischemia model

Findings from large cohort analysis can guide ongoing quality improvement initiatives

Insights and what’s next for the first U.S. mobile stroke unit to treat patients

Research to test clinical efficacy and cost-effectiveness versus standard-of-care rehab

Times to target blood pressure, CT and medication administration shorter than with EMS transport