Post hoc analysis of PROTECTED TAVR finds reduced stroke risk in the U.S. but not beyond
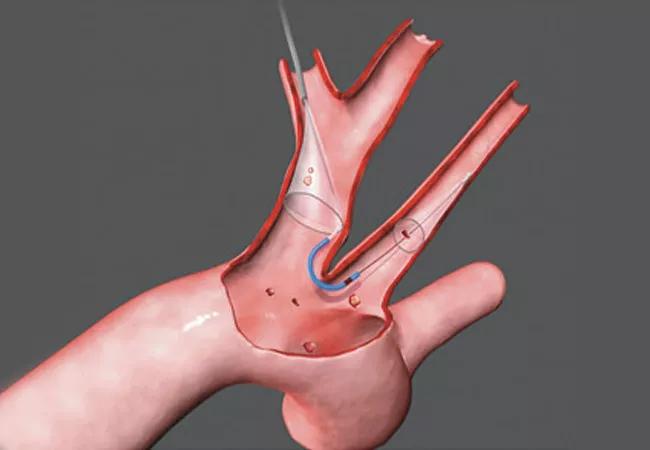
A new post hoc analysis of the PROTECTED TAVR trial suggests that cerebral embolic protection (CEP) during transcatheter aortic valve replacement (TAVR) may be more effective at reducing stroke risk in U.S. patients compared with patients treated outside the United States. While primary results of the PROTECTED TAVR trial showed no significant overall difference in stroke rates with CEP use, this exploratory analysis found relative reductions of 50% in stroke and 73% in disabling stroke among U.S. patients treated with a CEP device.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The findings — presented as a late-breaking clinical trial at the Transcatheter Cardiovascular Therapeutics conference (TCT 2024) and simultaneously published online in JAMA Cardiology — offer a potential explanation for conflicting evidence about CEP effectiveness.
“Regional differences in patient characteristics and procedural practices may influence the effectiveness of cerebral embolic protection in reducing TAVR-related stroke,” says Cleveland Clinic Cardiovascular Medicine Chair Samir Kapadia, MD, who presented the findings and served as co-principal investigator. “However, these results are only hypothesis-generating, and further studies are required to explore whether benefit from cerebral embolic protection in TAVR may be greater in specific subgroups.”
The analysis stems from ongoing uncertainty about the role of CEP in TAVR. While stroke remains a dreaded complication of TAVR, occurring in nearly 2.5% of cases, adoption of CEP devices has been limited, with utilization rates of approximately 13% as of 2019. This relatively low uptake reflects conflicting evidence about the devices’ effectiveness, including primary results from PROTECTED TAVR, which failed to meet its primary endpoint of reducing overall stroke despite showing a significant reduction in disabling stroke.
The current analysis examined outcomes from the prospective PROTECTED TAVR trial — which randomized 3,000 patients to TAVR with or without CEP using the Sentinel™ CEP device — to assess for any regional differences in outcomes between the 1,833 patients treated at 37 U.S. sites and the 1,167 patients treated at 14 sites in Europe and Australia.
Advertisement
The analysis found significant differences between the U.S. and non-U.S. cohorts in several characteristics: U.S. patients were younger, were more likely to be male, had lower rates of atrial fibrillation, and had higher rates of coronary artery disease, diabetes mellitus and peripheral vascular disease. They also were more likely to receive balloon-expandable valves (77.7% vs. 42.4% in non-U.S. patients).
Key outcome findings (based on follow-up at discharge or 72 hours after TAVR) included the following:
The analysis also revealed differences in patterns of post-stroke care. After experiencing a stroke, 36% of U.S. patients were discharged within 72 hours, compared with 22% of non-U.S. patients. U.S. patients also were more likely to be discharged home, whereas their non-U.S. counterparts were more commonly transferred to another hospital.
“Our findings suggest a trend toward protection from overall stroke with cerebral embolic protection in the U.S. cohort but not in the non-U.S. cohort, which could be explained by differences in patient factors and procedural practices by region,” Dr. Kapadia observes.
Advertisement
He and his co-authors acknowledge that these subgroup analyses were not prespecified and that the original trial was not powered to detect differences between geographic regions. They note, however, that their findings align with recent data from the TVT Registry (Circ Cardiovasc Intervent. 2024;17[9]:e013697) showing significant reductions in overall and disabling in-hospital stroke with CEP use among a sample of over 400,000 U.S. TAVR patients in real-world practice.
Uncertainty around CEP was heightened by a communication from the United Kingdom’s BHF PROTECT-TAVI trial issued just days before this presentation of the PROTECTED TAVR analysis at TCT 2024. The communication announced that recruitment into the multicenter randomized BHF PROTECT-TAVI trial had been terminated on Oct. 9, 2024, after an interim analysis determined there was “little prospect of demonstrating a benefit in the primary endpoint” and that a potential for harm could not be ruled out. The trial was assessing TAVR plus CEP (with the same CEP device used in PROTECTED TAVR) versus TAVR alone, with a primary endpoint of stroke incidence within 72 hours of TAVR or before hospital discharge (if sooner). The interim analysis included data from 7,490 randomized patients.
Detailed results from BHF PROTECT-TAVI will be presented at the American College of Cardiology Scientific Session in March 2025 (ACC.25). An analysis of combined findings from BHF PROTECT-TAVI and PROTECTED TAVR will also be presented at ACC.25, Dr. Kapadia says.
Advertisement
Additional insights into the effects of CEP are expected from a prospective meta-analysis that has been registered with the National Institute for Health Research. Moreover, several other CEP devices are under clinical investigation.
“More research is needed to understand differences in CEP effectiveness like those we have found in PROTECTED TAVR, whether based on region or patient characteristics or procedural factors,” Dr. Kapadia concludes. “Particularly welcome are efforts to develop predictive models to identify subgroups that may be most likely to benefit from cerebral embolic protection, enabling more targeted utilization.”
“There are probably many factors, some measured and some unmeasured, that contribute to regional differences in CEP efficacy, as shown in the current analysis,” adds Amar Krishnaswamy, MD, Section Head of Interventional Cardiology, who was not involved in the study. “So, at the current time, we continue to use cerebral embolic protection in all TAVR patients at Cleveland Clinic because we have noted a clear reduction in stroke in our experience with its use. There are also a number of new CEP devices at various stages of evaluation that may provide a better understanding of CEP use moving forward.”
The study was supported by Boston Scientific, which markets the device studied in PROTECTED TAVR. Dr Kapadia reported serving as principal investigator of studies sponsored by Boston Scientific without financial conflict.
Advertisement
Advertisement

TVT Registry analysis could expand indication to lower surgical risk levels

Patient series and bench validation support efficacy and safety of CLEVE procedure

In the wake of NOTION-3 findings, a strong argument for physician judgment remains
Analysis of STS/ACC TVT Registry finds greatest benefit in patients with prior stroke

TAVR explant demands multidisciplinary expertise

How our HVTI Advisory Services team facilitated swift improvements for an allied health organization
Support for a TAVR-first approach in patients with concurrent valve and coronary disease
Five-year data demonstrate convergence of outcomes from years 1 to 5